Editors’ Picks: June Highlights from the AACR Journals
For June, the editors of the AACR’s 10 journals chose to spotlight a characterization of an investigational KRAS inhibitor, an analysis of rural cancer prevention agencies, and results from multiple clinical trials. This edition of Editors’ Picks also features four studies on diverse immunotherapies. As always, the selected articles are freely available online for a limited time.
Journal: Molecular Cancer Therapeutics
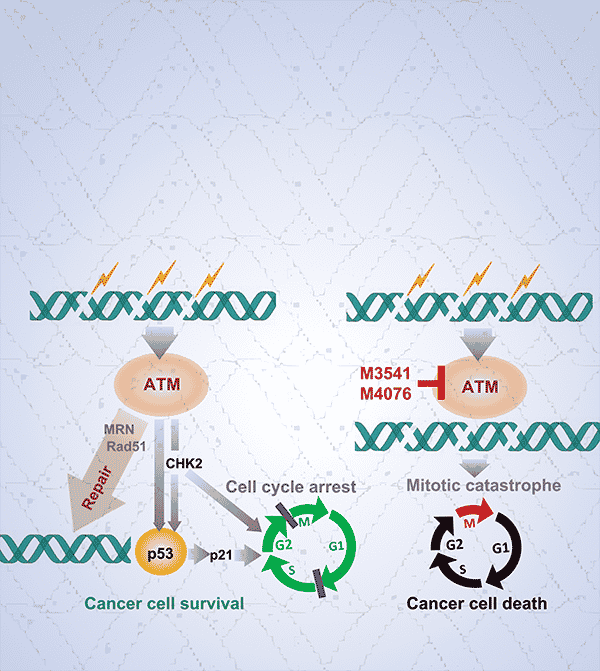
Many cancer treatments, including radiation and certain forms of chemotherapy, induce double-strand breaks in the DNA of cancer cells. These breaks, however, are typically repaired by cellular machinery, including the kinase ataxia telangiectasia-mutated (ATM), which has become an attractive target to enhance the efficacy of DNA-damaging therapies. In this study, the researchers described a new class of optimized ATM inhibitors, including the compounds M3541 and M4076. They demonstrated that, in several cancer cell lines, both compounds specifically inhibited ATM and blocked increases in ATM activity caused by radiation or the chemotherapy bleomycin. Treatment with either compound in combination with radiotherapy also increased the number of double-strand breaks, decreased cell growth due to cell cycle arrest, and reduced the formation of clonal colonies, as compared with either treatment alone. In mouse models harboring cell xenografts, both compounds drove robust tumor regression when combined with radiation, the chemotherapy cisplatin, or both. M4076 was selected for further development due to its improved solubility and potency, and it was shown to enhance the activity of other DNA damaging agents, including topoisomerase inhibitors and PARP inhibitors. The researchers suggest that combining M4076 with other DNA-damaging therapies may improve patient prognosis in a variety of tumor types, and they are currently testing M4076 in a phase I clinical trial. This article was highlighted and featured on the cover of the June issue.
Journal: Clinical Cancer Research (June 1 issue)
Increased activation of the PI3K protein promotes tumorigenic processes, such as cell proliferation, migration, and angiogenesis. As such, there is interest in therapeutically targeting PI3K; however, PI3K inhibitors developed to date have led to toxicity due to nonspecific targeting of multiple PI3K isoforms. Here, the authors reported results from a phase I clinical trial that evaluated AZD8186, an investigational PI3K inhibitor that selectively targets the PI3Kβ isoform, alone or in combination with abiraterone acetate plus prednisone or with vistusertib. The trial enrolled 161 patients with solid tumors. AZD8186 was well tolerated as monotherapy or in combination, with gastrointestinal symptoms representing the most common adverse events. There was preliminary evidence of antitumor activity in patients treated with AZD8186 monotherapy or with either combination. The authors suggest that the data support further evaluation of AZD8186 in larger clinical trials. This study was highlighted in the June 1 issue.
Journal: Clinical Cancer Research (June 15 issue)
Inhibitors of the oncogenic ALK protein have improved the survival of some patients with ALK+ non-small cell lung cancer (NSCLC), but the prognosis for patients with metastases to the brain and leptomeninges remains poor. The ALK inhibitor ceritinib (Zykadia) has demonstrated durable responses in some patients who experienced disease progression on or following treatment with another ALK inhibitor. In this phase II clinical trial, the researchers sought to evaluate the efficacy of ceritinib specifically in patients with brain or leptomeningeal metastases. Eighteen patients with leptomeningeal metastases and 138 patients with brain metastases (subdivided into treatment arms based on prior exposure to ALK inhibitors and radiotherapy) were treated with 750 mg of ceritinib daily until disease progression. After a median of 33.84 months, the whole-body overall response rate was 59.1 percent for patients with no prior ALK inhibitors or radiotherapy, 30.0 percent for patients with prior ALK inhibitors, 50.0 percent for patients with prior radiotherapy, 35.7 percent for patients with prior ALK inhibitors and radiotherapy, and 16.7 percent for patients with leptomeningeal metastases. Intracranial overall response rates were similar and remained highest for patients with no prior ALK inhibitors or radiotherapy. The authors of this study suggest that ceritinib may provide a durable benefit to patients with intracranial metastases of ALK+ NSCLC. This study was highlighted in the June 15 issue, and a related commentary is available here.
Journal: Blood Cancer Discovery
Chimeric antigen receptor (CAR) T-cell therapy has led to durable responses in many patients with recurrent/relapsed large B-cell lymphoma (LBCL); however, this treatment is associated with severe, sometimes fatal inflammatory toxicities, such as immune effector-cell associated neurotoxicity syndrome (ICANS). Since clonal hematopoiesis is a known driver of systemic inflammation, the authors of this study hypothesized that clonal hematopoiesis might play a role in the occurrence and severity of inflammatory toxicities in patients with LBCL who are treated with CAR T-cell therapy. To test this hypothesis, the researchers examined peripheral blood samples from 114 patients with recurrent/relapsed LBCL. Clonal hematopoiesis was detected in 42 pre-treatment samples. ICANS was found to be higher in patients with clonal hematopoiesis than those without, with the greatest ICANS severity observed among patients with clonal hematopoiesis in the DNMT3A, TET2, and ASXL1 genes. The incidence of therapy-related myeloid neoplasms after CAR T-cell therapy was also higher among patients with clonal hematopoiesis. The authors conclude that clonal hematopoiesis is a driver of toxicities associated with CAR T-cell therapy.
Journal: Cancer Discovery
Small molecule covalent inhibitors of the KRAS oncogenic variant KRASG12C have shown antitumor activity against KRASG12C-mutated cancers, providing a new therapeutic option for patients whose tumors carry this mutation. However, resistance can emerge through multiple mechanisms, including RAS pathway reactivation. In this study, the authors report the discovery and characterization of JDQ443, a structurally distinct KRASG12C inhibitor with a novel binding mode, which may present clinical advantages, especially in combination strategies. In vitro studies showed that JDQ443 potently inhibited KRASG12C-dependent signaling and proliferation in KRASG12C-mutated cell lines, including those carrying G12C/H95 double mutations. In vivo, JDQ443 treatment of tumor xenografts derived from KRASG12C-mutated cell lines (CDXs) inhibited tumor growth in all models in a dose-dependent manner. In patient-derived xenografts, the activity of JDQ443 was enhanced by combination with inhibitors of SHP2, MEK, and CDK4/6. The efficacy of the combination of JDQ443 and the SHP2 inhibitor TNO155 was maintained at lower doses of either agent in CDX models, suggesting synergy. A clinical trial was recently initiated to evaluate the safety and efficacy of JDQ443 as a monotherapy and in combination with TNO155 and PD-1 blockade in patients with KRASG12C-mutated solid tumors. The authors highlighted two cases from this trial, with activity of JDQ443 as monotherapy in a patient with advanced non-small cell lung cancer and in combination with TNO155 in a patient with advanced duodenal cancer that had not responded to prior chemotherapy. This article was highlighted and featured on the cover of the June issue.
Journal: Molecular Cancer Research
Delivery of IL-12-encoding plasmids into tumors via electroporation has been shown to induce localized IL-12 expression and regression of treated and distant tumors with minimal toxicity. Intratumoral IL-12 expression recruits both cytotoxic and immune-suppressing T cells into the tumor microenvironment. In this study, the authors examined whether combining IL-12 therapy with an intratumoral membrane-anchored anti-CD3 antibody would promote a more immune-activating tumor microenvironment. They found that combined electroporation of plasmids encoding IL-12 and membrane-anchored anti-CD3 enhanced effector cytokine production (as measured by interferon γ and granzyme B staining), increased T-cell cytotoxicity and proliferation, and reduced immune suppression within the tumor microenvironment. This led to greater regression of treated and non-treated tumors in mouse models. The combination of IL-12 and membrane-anchored anti-CD3 also improved the antitumor activity of tumor-infiltrating lymphocytes isolated from a patient who had progressed while receiving immune checkpoint inhibitor therapy. The authors conclude that the addition of membrane-anchored anti-CD3 may enhance IL-12 therapy. This article was highlighted in the June issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
The incidence of several cancer types with modifiable risks, such as tobacco use and obesity, is higher in rural areas of the United States than in urban areas. Informal multisector collaborative networks for cancer prevention and control are increasingly common but not well studied in rural areas. The authors of this study conducted a survey of informal multisector networks among agencies that address cancer risk in areas of rural Missouri and Illinois and analyzed the data with a method called social network analysis. The survey analyzed contact frequency, collaborative activities, and referrals. The study found that the networks were used more for exchanging information than for more time-intensive collaborative activities such as co-developing and sustaining services and programs and co-developing and sharing resources. Furthermore, they tended to be decentralized, and, while some agencies had more connections than others, the collaborative activities were not dependent on them to hold the network together. The researchers disseminated their findings from the network survey to practitioners, health and social service agency staff through infographics and online interactive platforms and collected feedback on uses of network findings. Users found the information useful for identifying collaboration gaps, enhancing or strengthening certain collaborative relationships, and showing network strengths to funders. According to the authors, the information provided by network analysis can help rural areas better leverage their resources to address cancer risk. This study was highlighted and featured on the cover of the June issue.
Journal: Cancer Research Communications
Aggressive gliomas are marked by large hypomethylated regions of the genome. Increased methylation of CpG islands within these regions is driven by mutations in isocitrate dehydrogenase (IDH), which are associated with better prognosis. However, IDH mutations are rare in glioblastoma, the most common and aggressive form of glioma, leading researchers to search for pharmacological ways to increase CpG methylation in these patients. In this phase I clinical trial, the researchers tested the efficacy of the methyl donor L-methylfolate (LMF), in combination with the standard-of-care drugs temozolomide (Temodar) and bevacizumab (Avastin), in 14 patients with IDH-wild type gliomas. Patients were treated once daily with 15 mg or twice daily with 30, 60, or 90 mg of LMF, plus 10 mg/kg bevacizumab every two weeks and a five-day regimen of 150 mg/m2/day of temozolomide per 28-day cycle. A retrospective control cohort of 50 patients treated with temozolomide and bevacizumab was assembled from patient medical records. No dose-limiting toxicities were observed. During the trial, 43 percent of patients experienced a partial response, 43 percent of patients experienced stable disease, and 14 percent of patients experienced disease progression. The overall survival and progression-free survival were not significantly different between the LMF cohort and the control cohort; the authors suggest a larger trial is needed to further assess survival benefit. Six patients donated their brains upon death, and their post-treatment tumors had significantly higher methylation compared with their pre-treatment tumors and with control tumors from publicly available data. While LMF treatment did not significantly improve survival in this trial, the authors suggest that its ability to reprogram DNA methylation may prove useful for future studies aimed at epigenomic therapy.
Journal: Cancer Prevention Research

In women with high risk for breast cancer, chemoprevention with antiestrogens, including selective estrogen receptor modulators and aromatase inhibitors, has been shown to lower the chances of disease development. However, the uptake of breast cancer chemoprevention is low, especially among racial/ethnic minorities, due to lack of routine risk assessment, inadequate knowledge about chemoprevention, and concerns about side effects. The authors of this article have previously developed web-based decision-support tools to help patients and providers navigate risk assessment and decision-making regarding chemoprevention. In this study, they described different strategies they implemented to identify and recruit participants for a randomized controlled trial to assess whether the web-based support tools could increase chemoprevention uptake among high-risk women. Some of the strategies relied on using existing databases and electronic health records to identify women previously enrolled in a clinical study of breast cancer risk; women with elevated breast cancer risk estimated through a risk-assessment tool; and women diagnosed with atypical hyperplasia or lobular carcinoma in situ. In addition, participants could be referred through clinical encounters with healthcare providers enrolled in the trial or by self-referral through flyers and online materials. Through these strategies, the study enrolled a racially/ethnically diverse cohort of 300 women, more than 40 percent of whom belonged to underrepresented minority groups. The majority (44.7 percent) of participants were recruited through discussion and referral from a health care provider and 27.3 percent came from a prior study cohort. According to the authors, the strategies and observations described in this study may be useful for future breast cancer chemoprevention trials to increase recruitment. This article was featured on the cover of the June issue.
Journal: Cancer Immunology Research

Immune checkpoint blockade with monoclonal antibodies is a powerful anticancer therapy, but most patients do not benefit from this approach, and many who respond also develop immune-related adverse events. To overcome these limitations, the authors of this study engineered tumor-specific CD8-positive T cells to deliver PD-L1-blocking nanobodies to the tumor site. Nanobodies are small antigen-binding antibody fragments with a short half-life in the blood stream. In a mouse colon tumor model, this approach improved tumor control compared with injection of PD-L1-specific antibody combined with adoptive cell transfer of tumor-targeting T cells. The researchers also observed that systemically injected anti-PD-L1 antibodies had limited tumor penetration leading to antigen occupancy in the periphery but not in the tumor microenvironment. In contrast, nanobody-secreting T cells migrated to the tumor sites and allowed specific intratumoral delivery of nanobodies, while also reducing systemic exposure. According to the authors, these results indicate that poor antibody penetration is a critical barrier to efficacy of immune checkpoint blockade, and nanobody-secreting T cells may represent a promising strategy to improve tumor penetration and minimize the risk of immune-related adverse events. This article was featured on the cover of the June issue.
Journal: Cancer Research (June 15 issue)
Mutations in the gene PIK3CA (and its resulting protein product PI3K) can drive oncogenesis in many tumor types, including estrogen receptor-positive breast cancer. While the effects of PI3K on cell growth, survival, and metabolism have been well characterized, PI3K also regulates RNA splicing—the process by which an immature transcript is trimmed into the final instructions encoding a protein. In this study, the researchers sought to characterize transcriptome-wide changes in splicing mediated by PIK3CA mutations and PI3K inhibitors. In human and mouse cell line models, improper splicing events were significantly increased in cells expressing mutant PIK3CA and returned to near-normal levels after treatment with a PI3K inhibitor. The researchers selected two alternatively spliced oncogenes—focal adhesion kinase (FAK) and AFMID—and identified the most common splice variants induced by PIK3CA mutation. Knockdown of the AFMID alternative splice variant by siRNA or re-expression of the wild type FAK or AFMID splice variants decreased cell proliferation and migration. The researchers also examined global splicing patterns in patients with PIK3CA mutations enrolled in a phase I clinical trial of the PI3K inhibitor inavolisib and found over 4,000 alternative splicing events between pretreatment and posttreatment samples, including events in FAK and AFMID. These data suggest that splicing may serve as another mechanism by which PIK3CA mutants drive cancer growth and migration. A commentary on this study is available here.
Journal: Cancer Research (June 1 issue)
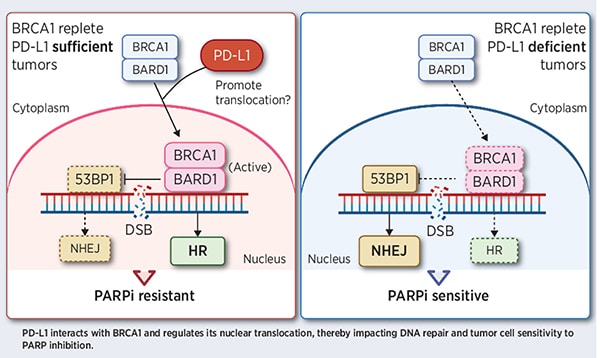
The immune checkpoint protein PD-L1 and the DNA repair protein PARP are each targeted by clinically available cancer therapies. The authors of this study examined potential interactions between the PD-L1 and PARP signaling pathways. Using cancer cell models, they found that PD-L1 promoted the nuclear accumulation of the BRCA1 DNA repair protein as well as DNA end resection, which is an early step in DNA repair by homologous recombination. Depletion of PD-L1 led to reduced DNA repair through the homologous recombination pathway and increased activation of the nonhomologous end-joining pathway, a phenotype typically seen in BRCA1-mutated cells. Furthermore, the researchers found that depletion of PD-L1 was synthetically lethal with PARP inhibition in some cancer cells with wild-type BRCA1, revealing PD-L1 ablation as a potential therapeutic strategy to sensitize cancers with wild-type BRCA1 to PARP inhibitors. Similar results were observed in mice when PD-L1 was genetically depleted, but not when it was inhibited with immune checkpoint blockade. Together, the results suggest that PD-L1 may have a role in BRCA1-mediated DNA damage repair independent of immune checkpoint function. A related commentary can be found here.