Hard-hitting Therapies for Soft Tissue Cancers: New Treatment Approaches for Sarcomas
Accounting for less than 1 percent of all newly diagnosed cancers in the United States each year, sarcomas are relatively rare. However, these cancers lead to death in approximately 35 percent of patients diagnosed with them.
Sarcomas can develop anywhere in the body in soft tissues such as muscle, fat, tendons, nerves, lymph nodes, or the tissue around joints. These contrast with carcinomas, a much more common form of cancer that develops in epithelial cells of various organs.
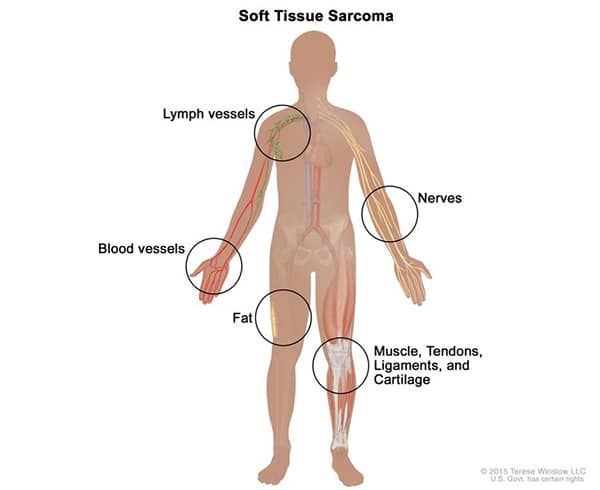
Sarcomas are frequently treated with surgery, which may be the only treatment required for early-stage, low-grade tumors. For more advanced sarcomas, however, additional therapies, such as chemotherapy, radiation, targeted therapy, or immunotherapy may be required.
Researchers continue to discover and evaluate novel strategies to treat various forms of sarcoma. Recent studies published in American Association for Cancer Research (AACR) journals and elsewhere report promising results for a number of these approaches.
Modulating the immune response
One treatment approach under investigation is utilizing immunotherapy to promote antitumor activity by the immune response. In a recent phase II clinical trial published in the AACR journal Clinical Cancer Research, researchers evaluated an investigational anti-PD-L1 immune checkpoint inhibitor, TQB2450, in combination with the anti-angiogenesis drug anlotinib in 30 patients whose metastatic soft tissue sarcomas did not respond to prior chemotherapy. Anlotinib is approved to treat sarcomas in China, where the trial was conducted.
Clinical responses were observed in approximately 37 percent of patients. The median progression-free survival was 7.85 months, and over 69 percent of patients were alive one year after starting treatment. Among the 12 patients with alveolar soft part sarcoma (ASPS), 75 percent had a clinical response, and all 12 patients had some level of disease control in response to the therapy. In contrast, only 11 percent of patients with other types of sarcoma had a clinical response. The researchers concluded that combining TWB2450 with anlotinib was effective against ASPS, suggesting that dual inhibition of immune checkpoint activity and angiogenesis may be a promising treatment strategy for this sarcoma.
Another clinical trial also demonstrated the potential of immune checkpoint inhibition to treat sarcoma. This trial, the results of which were published in Lancet Oncology and summarized in the AACR journal Cancer Discovery, evaluated the anti-PD-1 therapy pembrolizumab (Keytruda) in patients with Kaposi sarcoma, a cancer that typically develops in immunocompromised individuals. Reducing immunosuppression with other therapies has shown promise against this disease, but the impact of immunotherapy remains undefined.
The investigators observed that 71 percent of the 17 patients enrolled in the trial had a clinical response, with 10 partial and two complete responses. These results suggest that pembrolizumab could be effective for patients with Kaposi sarcoma, although studies in a larger group of patients are needed.
Another trial, published in Clinical Cancer Research, examined the immunomodulatory drug lenalidomide (Revlimid) in patients with Kaposi sarcoma who were positive for HIV. Lenalidomide activates various immune cells while suppressing the secretion of certain pro-inflammatory cytokines. Beyond these immunomodulatory functions, lenalidomide also blocks angiogenesis and induces cell death.
In the phase I/II trial, lenalidomide treatment led to clinical responses in 60 percent of evaluable patients. Immune analyses using patient samples demonstrated an increased number of T regulatory cells and a reduction in inflammatory cytokines, consistent with lenalidomide’s known effects. The authors concluded that lenalidomide could be a viable treatment option for patients with Kaposi sarcoma. Several follow-up clinical trials of lenalidomide, alone or in combination with other therapies, have been initiated for patients with Kaposi sarcoma.
Targeting gene expression in Ewing sarcoma
Ewing sarcoma is an aggressive cancer that primarily affects adolescents. In most cases, the disease is driven by a fusion of the EWS and FLI1 genes, EWS-FLI1, which results in extensive chromatin remodeling and widespread transcriptional dysregulation that promotes tumor formation and metastasis. EWS-FLI1 is, therefore, a potential therapeutic target.
Lurbinectedin (Zepzelca) selectively inhibits EWS-FLI1 by relocating it to the nucleolus and has been shown to delay tumor growth in mouse models of Ewing sarcoma. A recent phase II clinical trial, the results of which were published in Clinical Cancer Research, evaluated lurbinectedin in patients with relapsed Ewing sarcoma.
Approximately 57 percent of patients in the trial experienced some level of disease control, and 14 percent had a clinical response to lurbinectedin. The median duration of response was 4.2 months, and patients survived for a median of 12 months. Due to the antitumor activity observed in this trial, the authors of the study suggested that lurbinectedin could be effective in conjunction with other therapies.
In addition to inhibiting EWS-FLI1 itself, targeting regulation of the fusion protein could be another therapeutic approach. A recent study from Clinical Cancer Research uncovered a new mechanism by which EWS-FLI1 activity is regulated. It was previously known that high levels of EWS-FLI1 activity promote proliferation, whereas low activity leads to mesenchymal features and metastatic proclivity. In addition, prior studies had shown that expression of the HOXD13 transcription factor in Ewing sarcoma promotes metastasis, but the underlying mechanism remained unclear.
In this study, researchers determined that HOXD13 was enriched at known EWS-FLI1 binding sites, where it impacted expression of EWS-FLI1-regulated genes, including activation of genes normally repressed by EWS-FLI1. Activation of these genes led the cells to adopt a mesenchymal state that would typically be suppressed by the EWS-FLI1 fusion protein. The authors concluded that opposing functions of HOXD13 and EWS-FLI1 influence cell states and the metastatic potential of Ewing sarcoma. Understanding this intricate relationship could provide additional therapeutic opportunities.
Combining a tyrosine kinase inhibitor with chemotherapy for uterine sarcoma
Cabozantinib is an inhibitor of multiple tyrosine kinases, including several expressed in sarcomas. As such, researchers are interested in examining its potential to treat patients with various sarcomas. Prior research suggested that cabozantinib could be an effective therapy for uterine sarcoma, both alone and in combination with other therapeutics. Temozolomide is a chemotherapeutic that kills cancer cells by damaging DNA. It has demonstrated antitumor activity against several sarcomas, including some activity against uterine sarcomas.
In a recent study published in Clinical Cancer Research, researchers used preclinical models of uterine sarcoma to evaluate the impact of combining these two therapies.
They found that combining cabozantinib and temozolomide significantly decreased growth and viability and increased apoptosis in established cell lines and patient-derived sarcoma cells, compared with cells treated with each drug individually. Furthermore, the combination led to a synergistic reduction in tumor size in a xenograft mouse model. Molecular analyses indicated that the combination led to a synergistic inhibition of AMPK phosphorylation, providing a potential mechanism of action.
Based on these results, the authors concluded that cabozantinib in combination with temozolomide showed promise against uterine sarcoma and suggested that the combination be evaluated in a clinical trial.
Although sarcomas are relatively rare, they can be devastating for the patients who are diagnosed with them. Ongoing research will continue to uncover new treatment strategies to improve outcomes for those affected by these cancers.