Crystal Mackall: Expanding the Reach of CAR T-cell Therapy
For the sixth time since 2015, researchers, clinicians, drug developers, patient advocates, and other stakeholders convened at the International Cancer Immunotherapy Conference (CICON) to discuss the latest developments in immunotherapy research.
This year’s conference—the first since the COVID-19 pandemic began—was held September 28-October 1 in New York City and was hosted by the American Association for Cancer Research (AACR), the Cancer Research Institute, and the European Network for Cancer Immunotherapy.
Front and center at CICON were major advances in chimeric antigen receptor (CAR) T-cell therapy, as showcased by immunologist and CAR T-cell pioneer Crystal Mackall, MD, FAACR, in her keynote address to attendees. Mackall is a professor of pediatrics and medicine at the Stanford University School of Medicine.
CAR T-cell therapy is a form of cell-based immunotherapy in which a patient’s T cells are extracted, engineered to target cancer cells, expanded, and infused back into the patient. The technology has revolutionized cancer therapy, leading to improved survival for many patients with blood cancers. Despite its promises, CAR T-cell therapy also has several limitations. Responses are frequently short-lived, and disease recurrence is common. Moreover, CAR T-cell therapy has thus far been ineffective against solid tumors in the clinic.
Cancers develop resistance to CAR T-cell therapy through various mechanisms, including loss of the target antigen (antigen escape), reducing antigen density, or switching to a related but phenotypically different disease that does not express the tumor antigen (lineage switch). Understanding how cancer cells mediate resistance and identifying strategies to prevent it will be key to improving patient outcomes.
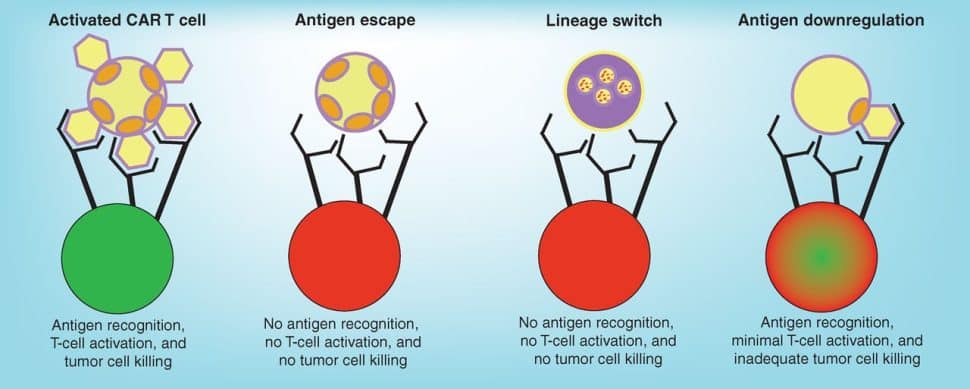
Several factors have made solid tumors a particularly difficult target for CAR T-cell therapy. For one, these cancers tend to have heterogeneous expression of tumor antigens, and many of these antigens are also expressed on normal cells, increasing the risk of off-tumor activity. Additionally, the physical barriers posed by the surrounding stroma limit access to the tumor, and various components of the tumor microenvironment suppress immune activity.
Despite these challenges, Mackall noted that researchers are starting to see signals of activity in solid tumors. In her keynote lecture, she described some of the innovative approaches developed and tested by her research team to expand the reach of CAR T-cell therapy.
In the first vignette, Mackall shared encouraging results from a phase I clinical trial testing CAR T-cell therapy in patients with diffuse midline glioma, a deadly form of brain cancer. The CAR T cells evaluated in this trial were designed to target a protein called GD2, which Mackall and colleagues previously found to be overexpressed in a subset of these solid tumors.
Using preclinical mouse models, Mackall’s team previously showed that GD2-targeted CAR T cells effectively cleared gliomas, and that toxicity could be mitigated by incorporating a co-stimulatory domain or by transiently exposing CAR T cells to a chemical inhibitor of CAR signaling. Separately, Mackall and colleagues determined that intracerebroventricular administration of CAR T cells in mice led to greater efficacy against brain tumors with fewer toxicities compared with systemic administration.
Building off these preclinical findings, the researchers developed anti-GD2 CAR T cells with a 4-1BB co-stimulatory domain, which were initially administered systemically to patients with diffuse midline glioma, followed by intracerebroventricular administration for subsequent doses.
Mackall reported that three of the four patients who received the treatment experienced impressive responses, which were sustained by regular administration of the anti-GD2 CAR T cells. She noted that the success of their approach was due to targeting a highly expressed tumor antigen, delivering the CAR T cells to the tumor site, and repeated dosing to overcome T-cell dysfunction and poor expansion.
Curiously, two patients experienced disease relapse even though their tumors continued to express GD2, suggesting that factors beyond the target antigen contributed to relapse. Sequencing of cells from the cerebrospinal fluid revealed that recurrence correlated with increased levels of T regulatory (Treg) cells.
Underscoring the significance of Treg cells, Mackall discussed another study in which Treg cells were found to be associated with lymphoma progression. In this study, researchers utilized single-cell proteomics to profile circulating CD19-targeted CAR T cells after infusion into patients with lymphoma. They found that CAR T cells from patients with disease progression were more likely to express markers of Treg cells, a finding echoed in a similar study from Harvard researcher Marcela Maus, MD, PhD.
T-cell exhaustion is another means by which CAR T cells lose their antitumor potency. The third study presented by Mackall uncovered a novel approach to circumvent exhaustion and increase CAR T-cell potency. Mackall and colleagues showed that CD39+/CD8+ exhausted CAR T cells were phenotypically and transcriptionally similar to Treg cells, and that exhaustion in this subset of CAR T cells was associated with the presence of adenosine in the tumor microenvironment. Overexpression of adenosine deaminase, which converts adenosine to inosine, on CD39+/CD8+ CAR T cells prevented exhaustion and promoted a stem-like phenotype. This effect could be recapitulated by manufacturing CAR T cells in the presence of inosine, which enhanced antitumor potency, increased stemness, and diminished terminal differentiation. This suggested that the presence of inosine, rather than the loss of adenosine, was key to preventing T-cell exhaustion.
To identify additional genes that restrict the fitness of CAR T cells, Mackall and colleagues performed a genome-wide CRISPR knockout screen, which she summarized in the final story of her keynote address.
MED12 and CCNC, both components of the transcription-regulating Mediator complex, were top hits in the genetic screen. Further experimentation showed that MED12 deficiency enhanced T-cell fitness and antitumor immune activity and resisted CAR T-cell exhaustion. Interestingly, MED12 deficiency did not induce stemness—a shift from what is typically seen when T-cell fitness is enhanced.
Mechanistically, depletion of MED12 led to increased transcription of genes associated with antitumor T-cell activity, including the IL-2 receptor α gene. Accordingly, MED12-deficient T cells expressed higher levels of IL-2 receptor α and had greater sensitivity to IL-2, which would be expected to promote T-cell expansion and activity.
Mackall and colleagues also demonstrated that a small molecule inhibitor of CDK8/19 kinase activity partially mimicked MED12 deficiency in T cells, thus revealing a potential strategy to enhance T-cell fitness for immunotherapy.
The studies presented by Mackall highlight some of the varied approaches researchers are using to expand the reach of CAR T-cell therapy—from identifying new targets and mediators of relapse and exhaustion to utilizing different manufacturing protocols and modes of administration.
While challenges continue to limit the efficacy of CAR T-cell therapy for many patients and tumor types, ongoing research is driving progress, a point emphasized by Mackall.
“Even for someone like myself who was wowed by the first patient treated at the [National Cancer Institute] with CAR T-cell therapies, I didn’t think that their momentum or impact 10 years later would be as great as they’ve been,” she said. “I think there’s no reason CAR T cells can’t also work in solid tumors.”
On Monday, Oct. 17, Mackall was elected as a member of the National Academy of Medicine. Follow @AACR on Twitter to learn more about Mackall and the other AACR members chosen for this prestigious honor.
To learn about the real-world impact of CAR T-cell therapy, read a recent blog post about one patient’s journey with this immunotherapy. Additional resources on CAR T-cell therapy:
- Overview of CAR T-cell therapy in the AACR Cancer Progress Report 2022
- A perspective on next-generation CAR T-cell therapies in the AACR journal Cancer Discovery