Editors’ Picks: Highlights from AACR Journals
As the candy supply dwindles, and the Halloween sugar rush tapers off, get re-energized by the latest edition of Editors’ Picks, selected by the editors of the 10 AACR journals. Highlights from October include a new drug for glioblastoma, a comparison of prostate cancer genomes from patients of African and European ancestry, and a mechanism by which nerve cells form highways to help cancer cells metastasize.
As always, the selected articles are freely available for a limited time.
Journal: Cancer Research (October 15 issue)
A Distinct Chromatin State Drives Therapeutic Resistance in Invasive Lobular Breast Cancer
Most invasive lobular breast cancers are hormone receptor-positive, but they have poor responses to tamoxifen and are associated with worse patient outcomes than invasive ductal carcinomas. To understand the factors contributing to the low efficacy of tamoxifen in these cancers, researchers examined the epigenetic features in preclinical models and patient samples. They found that distinct epigenetic changes in invasive lobular carcinomas led to increased FOXA1 recruitment to chromatin; these changes were not observed in invasive ductal carcinomas. These changes were also associated with the generation of a FOXA1-estrogen receptor (ER) signaling axis that was unique to invasive lobular breast cancer and promoted alterations to gene expression linked to tumor progression and poor patient outcomes. Further, the researchers found that the FOXA1-ER axis resulted in prolonged chromatin-bound ER after tamoxifen treatment, which contributed to tamoxifen resistance. Targeting FOXA1 in invasive lobular carcinomas inhibited tumor growth. The authors conclude that a distinct chromatin state associated with invasive lobular carcinoma confers tamoxifen resistance and tumor progression. A related commentary can be found here.
Journal: Molecular Cancer Therapeutics
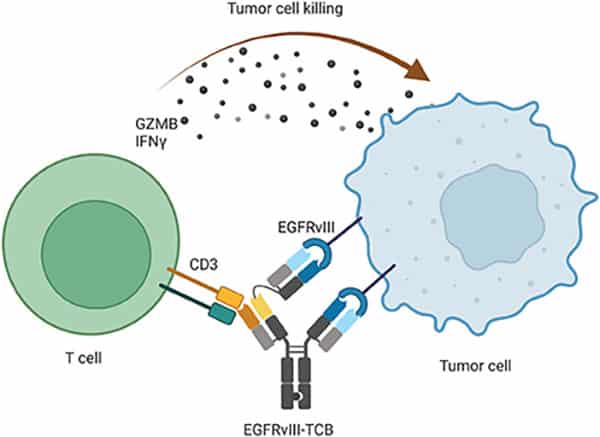
A Novel EGFRvIII T-Cell Bispecific Antibody for the Treatment of Glioblastoma
Glioblastoma is a lethal form of brain cancer that has thus far been difficult to treat with immunotherapies. T-cell bispecific antibodies (TCB) are designed to simultaneously bind T-cell receptors and tumor antigens to target T cells to cancer cells. Here, the authors designed and evaluated a novel TCB targeting the EGFRvIII mutation that is commonly found in glioblastoma, but not in normal cells. They observed that the EGFRvIII-TCB led to antitumor activity, T-cell activation, and cytokine secretion in patient-derived tumor models expressing the mutation. The investigational therapy also induced recruitment of T cells into intracranial tumors and led to tumor regression in animal models of glioblastoma. The authors conclude that these preclinical results support the evaluation of EGFRvIII-TCB in patients. A phase I clinical trial evaluating this therapy has been initiated. This article is highlighted and featured on the cover of the October issue.
Journal: Cancer Immunology Research
Treatment with immune checkpoint inhibitors can cause a variety of immune-related side effects, including skin rashes. Because treating rashes with high-dose, systemic steroids may affect the efficacy of immunotherapy, the authors of this study sought to understand the mechanisms underlying skin-related adverse events, to inform the potential development of new treatments. The researchers hypothesized that, similar to psoriasis, these immune-related skin conditions may be driven by Th-17 cells or other types of resident memory T cells (TRM)—memory cells that reside in tissues outside of immune organs. Skin samples from patients with immune-related adverse skin events and from patients with psoriasis had significantly more lymphocytes and TRM cells than normal samples, typically concentrated just below the epidermis. Using spatial transcriptomics, the researchers evaluated the gene expression profiles of two samples of posttreatment dermatitis and two samples of psoriasis and found that while psoriasis is characterized by Th-17 TRM cells, treatment-related adverse skin events are characterized by Th-1 TRM cells. The researchers also found high numbers of Th-1 TRM cells in seven samples of treatment-related colitis as compared with eight normal colon samples. Using RNA in-situ hybridization, the researchers confirmed that treatment-related dermatitis and colitis samples expressed high levels of immune checkpoint proteins such as PD-1, CTLA-4, and TIGIT, as well as inflammatory cytokines such as TNFα, CXCL9, and IFNγ. The latter was shown to be specifically expressed by TRM cells and could create a feed-forward loop that boosts TRM activity. The authors of the study suggest that these insights could help inform new topical and systemic therapies directed at Th-1 TRM cells. This study was also presented at the AACR Special Conference: Tumor Immunology and Immunotherapy, held in Boston October 21-24.
Journal: Molecular Cancer Research
The authors of this article previously reported that a signaling axis mediated by Exchange Protein directly Activated by cAMP (EPAC), a RAP small GTPase guanine nucleotide exchange factor (RAPGEF), is required for growth and survival of primary melanoma but not metastatic melanoma. In this study, they further investigated the mechanisms of action of EPAC in melanoma and the correlation between loss of dependency on EPAC signaling and tumor progression. Pharmacologic inhibition and genetic knockdown of EPAC resulted in slower tumor growth, cell cycle inhibition, and apoptosis in primary but not metastatic melanoma cells. Furthermore, EPAC inhibition caused downregulation of mTORC1 activity. The researchers also showed that EPAC regulated glycolysis, oxidative phosphorylation, and production of mitochondrial reactive oxygen species in primary melanoma cells. These findings suggest that EPAC signaling promotes cell survival and proliferation through activation of mTORC1 in primary melanoma and that melanoma cells progressively lose their dependency on EPAC signaling during tumor progression and metabolic adaptation. Analyses using The Cancer Genome Atlas revealed that high expression of the RAPGEF3 and RAPGEF4 genes, which encode for the EPAC isoforms EPAC1 and EPAC2, in primary melanoma predicted worse disease-free survival. According to the authors, this study suggests EPAC could represent a potential prognostic marker and target for prevention of melanoma progression. This study was highlighted in the October issue.
Journal: Clinical Cancer Research (October 15 issue)
EWS::FLI1 and HOXD13 Control Tumor Cell Plasticity in Ewing Sarcoma
The EWS::FLI1 fusion oncogene is the driver for most Ewing sarcomas, but the mechanisms regulating its transcriptional activity are unclear. In particular, it remains unknown why high EWS::FLI1 activity inhibits metastasis-promoting mesenchymal gene expression. Here, the authors examined whether the HOXD13 transcription factor, which has been shown to promote metastasis of Ewing sarcomas, contributed to EWS::FLI1 transcriptional activity. They found that depletion of HOXD13 altered expression of developmental gene programs and EWS::FLI1 transcriptional target genes. Furthermore, HOXD13 was found enriched at known EWS::FLI1 binding sites and regulated expression of EWS::FLI1-activated genes. Paradoxically, HOXD13 was also found to activate genes normally repressed by EWS::FLI1; HOXD13-mediated expression of these genes promoted mesenchymal and migratory cell states. The authors conclude that the transcriptional antagonism between HOXD13 and EWS::FLI1 helps define the mesenchymal state of Ewing sarcoma cells. This article was highlighted in the October 15th issue. A related commentary can be found here.
Journal: Blood Cancer Discovery
Little is known about the interactions between follicular lymphoma—a type of non-Hodgkin lymphoma arising from B cells—and other immune cells in the tumor microenvironment. In this study, the researchers performed single-cell RNA sequencing on lymph node biopsies from 20 patients with follicular lymphoma and three patients with benign reactive lymph nodes. They found that the majority of nonmalignant cells in lymphoma biopsies were T cells, including both CD4+ and CD8+ T cells in various cell states. Using marker genes identified in their single-cell analysis, the researchers analyzed bulk gene expression data from 1,269 follicular lymphoma samples and identified four distinct immune microenvironment subtypes based on T-cell abundance: naïve, warm, depleted, and intermediate. T-cell depleted microenvironments were associated with a poorer progression-free survival. The researchers found that common follicular lymphoma mutations, including CREBBP, were associated with deficits in antigen processing pathways, including the repression of MHCII expression. Tumors with loss of MHCII were more likely to have T-cell depleted or naïve microenvironments, marked by fewer cytotoxic CD4+ cells. Further, the immune microenvironment of tumors with low MHCII expression had a distinct gene expression profile from the microenvironment of tumors with high MHCII expression, including decreased expression of immunotherapy targets such as LAG3 and TIGIT. These data suggest a mechanism by which MHCII loss confers a selective advantage for follicular lymphoma cells and drives an immune-suppressive microenvironment. A related commentary is available here.

Journal: Cancer Research
Germline Missense Variants in CDC20 Result in Aberrant Mitotic Progression and Familial Cancer
In eukaryotic cells, mechanisms are in place to avoid aberrant separation of the chromosomes during mitosis. The anaphase promoting complex/cyclosome (APC/C) and its co-activator CDC20 are essential for mitotic progression. APC/C is inhibited by the spindle assembly checkpoint (SAC), which is triggered when the cells in mitosis are not ready to complete cell division. While overexpression of CDC20 is common in cancer, the consequence of CDC20 mutations has not been established. The authors of this article studied the genetic causes of familial malignant ovarian germ cell tumors (mOGCT) by sequencing blood DNA from five families with histories of mOGCT and identified two heterozygous missense mutations in the CDC20 gene in all affected individuals from two families. Further in vitro studies expressing these mutations (L151R and N331K) in cells showed that mutant CDC20 retained the APC/C co-activator activity but had impaired binding to BUBR1, a component of the SAC. As a consequence, cells underwent mitotic slippage, or exit from mitotic arrest, resulting in polyploidy. The researchers generated knock-in mice carrying the N331K mutation using CRISPR-Cas9 to assess the oncogenic ability of this mutation in vivo. While mice homozygous for the N331K variant were nonviable, heterozygous mice displayed accelerated development of Myc-induced lymphomas. A commentary related to this article is available here.
Journal: Cancer Prevention Research
By blocking the action of the inflammatory molecules leukotrienes, leukotriene receptor antagonists (LTRAs) are used to prevent and treat asthma. Recent studies have indicated that LTRAs inhibit carcinogenesis and cancer cell growth and might therefore be useful for cancer chemoprevention. In this article, the authors conducted a prospective study to assess the chemopreventive effect of the LTRA drug montelukast on the formation of aberrant crypt foci (ACF), early pre-cancerous lesions that are considered a surrogate marker of colorectal cancer. In this nonrandomized, open-label, controlled trial, 30 patients with colorectal ACF received montelukast daily for eight weeks, and 10 were allocated to the observation group. Patients underwent colonoscopy to assess the number of ACF at baseline and at the end of the treatment period. The mean number of ACF per patient significantly decreased by 2.4 in the group that received montelukast, including a reduction in both dysplastic and nondysplastic ACF, while there was no significant change in the observation group. Furthermore, there was a significant reduction in Ki-67, a proliferation marker, in tissue biopsies from the LTRA group but not the observation group. Adverse events from montelukast administration were mild and disappeared without any treatment. Although this was a short-term pilot trial, according to the authors, the results warrant further investigations to confirm the potential role of LTRAs in colorectal cancer interception. This article was featured on the cover of the October issue, and a related commentary is available here.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Because clinical trials are short-lived and monitor small numbers of patients, rare side effects and those that appear long after treatment are difficult to assess in new drugs. In 2008, the U.S. Food and Drug Administration (FDA) launched the Sentinel Distributed Database, a system that integrates data from hundreds of millions of electronic health records to explore the connections between diseases, treatments, and drug combinations. One possible use of the Sentinel system is to identify potential risks to patients based on their medical history. In this study, the researchers determined the average duration of data availability before and after each patient’s cancer diagnosis. Data from before a cancer diagnosis, the study authors claimed, could help researchers determine if certain medications increase a patient’s cancer risk, while data from after the diagnosis could illuminate any long-term effects of cancer treatments. The researchers identified 6,638,134 cancer cases to date, with the highest representation of breast, prostate, and lung cancers. The average duration of available data ranged, based on cancer type, from 4.0 to 4.6 years prior to cancer diagnosis and from 1.1 to 3.3 years after diagnosis. The researchers suggest that the availability of these data warrant additional studies on the prospective uses of the Sentinel system in cancer care. This study was highlighted and featured on the cover of the October issue.
Journal: Clinical Cancer Research
OLIG2 Is a Determinant for the Relapse of MYC-Amplified Medulloblastoma
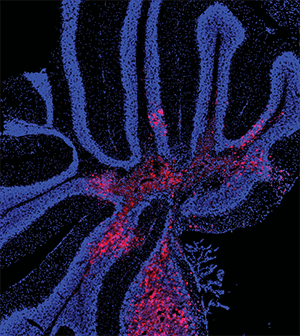
Medulloblastoma (MB) is the most common malignant pediatric brain tumor. A subtype of MB carrying MYC amplification or overexpression is highly aggressive and has poor prognosis despite intensive treatment. The OLIG2 transcription factor is highly expressed in MB and other gliomas and serves as a diagnostic marker for these tumors. In this study, the authors evaluated whether OLIG2 could be used as a biomarker for risk stratification of MYC-driven MB. Analysis of OLIG2 expression in a panel of mouse tumors, patient samples, and patient-derived xenografts showed that these tumors could be divided into an OLIG2-high subset and an OLIG2–low subset that had different responses to radiation: While OLIG2–low tumors were radiosensitive and rarely relapsed, OLIG2–high tumors were radioresistant and frequently developed recurrence. Further studies showed that irradiation of OLIG2–high tumors in vivo selected quiescent cells and reduced the number of OLIG2-expressing cells. However, tumors that progressed after irradiation consisted mostly of OLIG2-positive tumor cells, reflecting the composition of the original tumors. Furthermore, OLIG2 overexpression rendered OLIG2-low MYC-amplified MB cells resistant to radiation. Targeting OLIG2 with CRISPR or with the small molecule inhibitor CT-179 in combination with radiotherapy prevented the recurrence of OLIG2-high MYC-amplified tumors. This study revealed OLIG2 as a biomarker for high-risk, MYC-amplified MB, and suggests it could be targeted with CT-179 to prevent or delay recurrence after radiotherapy. This study was highlighted in the October issue and featured on the cover.
Journal: Cancer Discovery
Reprogrammed Schwann Cells Organize into Dynamic Tracks that Promote Pancreatic Cancer Invasion
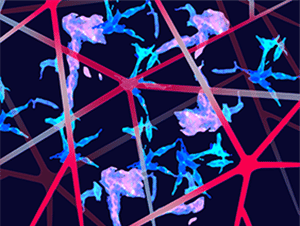
Many types of cancer, including pancreatic cancer, can use nerves as highways to escape the primary tumor and invade new sites. In this study, the researchers found that a subtype of Schwann cells—the most abundant cell type found in nerves—can be transcriptionally reprogrammed by pancreatic cancer cells to form linear tracks on which cancer cells can migrate. Gene signatures characteristic of these nonmyelinating Schwann cells correlated with poor prognosis in samples from patients with pancreatic cancer and were enriched for the activation of metastasis-driving pathways such as MAPK and PI3K signaling, epithelial-mesenchymal transition, and extracellular matrix reorganization. In cell cultures and human samples, Schwann cells expressing glial fibrillary acidic protein (GFAP) associated closely with pancreatic tumor cells and wrapped around them, forming chains of cancer cells the researchers called tumor-activated Schwann-cell tracks (TASTs). When cocultured Schwann and pancreatic cancer cells were driven to migrate through microchannels, the Schwann cells mechanically pushed and pulled the cancer cells through the chambers. The transcriptional reprogramming that stimulated this process was driven by aberrant activation of c-Jun within Schwann cells, which only occurred when Schwann cells were adjacent to cancer cells. Genetic knockout of c-Jun hindered the formation of TASTs and the migration of pancreatic cells in vitro and in a mouse model. These data highlight one way in which nerve cells can promote pancreatic cancer metastasis and position c-Jun inhibition as a potential therapeutic strategy. This article was featured on the cover of the October issue, and a commentary on this study is available here.
Journal: Cancer Research Communications
Black men have the highest rate of prostate cancer-related mortality in the United States. Insights into genetic ancestry could aid precision medicine efforts by uncovering therapeutic targets specific to patients with African ancestry. In this study, researchers compared DNA sequences from prostate tumors of Nigerian, African American, and European American patients. The analysis revealed that genetic variants in BRCA1 and other DNA repair genes—including some not previously known to impact prostate cancer—were similar between the Nigerian and African American prostate tumors. Furthermore, the researchers determined that the frequency of BRCA1 mutations increased with higher proportions of African genetic ancestry and decreased with lower proportions of African ancestry. “We identified unique variants in the BRCA1 gene that are not typically tested for in the clinic, and these variants were specific to African ancestry,” said the study’s senior author, Clayton Yates, PhD, in an AACR press release. “This suggests that different variants contribute to prostate cancer in European Americans compared with men with African ancestry.” The study was concurrently presented at the 15th AACR Conference on the Science of Cancer Health Disparities in Racial and Ethnic Minorities and the Medically Underserved. Read more about this study, as well as additional research examining the role of genetic ancestry in cancer, in a recent blog post.



