Heart Health: Mitigating Collateral Damage From Immunotherapy
The immune system is designed to operate like a well-oiled machine.
With a myriad of distinct cell types, a handful of specialized organs, and a network of watchtowers spread throughout the body, the immune system must strike a delicate balance. Too little activity, and it potentially exposes the body to harm by pathogens. Too much activity, and it risks turning against its host.
A system of checks and balances keeps the immune system working efficiently, but cancer cells can manipulate those signals to promote their own survival. For example, they can bombard immune cells with signals that prevent them from recognizing and destroying the tumor.
Immune checkpoint inhibitors (ICIs), a class of immunotherapeutics that suppresses these signals and enables the immune system to attack cancer cells, have been shown to improve patient prognosis in a variety of cancer types. ICIs have become a mainstay of cancer immunotherapy, benefiting thousands of patients each year. However, turning off the immune system’s natural brakes can cause unintended consequences in other parts of the body.
In rare cases, the overactive immune cells can damage the patient’s muscles, including those in the heart and respiratory system. Swelling of the heart (myocarditis) and damage to skeletal and respiratory muscles (myotoxicity) can be life-threatening, necessitating rapid and precise treatment. Unfortunately, with current therapeutic strategies, the mortality rate from these conditions remains high.
In a study published recently in Cancer Discovery, a journal of the American Association for Cancer Research (AACR), Joe-Elie Salem, MD, PhD, and colleagues proposed a new treatment regimen for ICI-related myocarditis, which dramatically decreased mortality for patients with the condition. Salem, a professor at Sorbonne Université, and executive assistant director of one of France’s Clinical Investigation Centers focused on cardio-metabolism, also provided an overview of how the toxicity occurs and why current treatments may fall short.
How ICI Works (Sometimes Too Well)
Cancer cells take advantage of several immune checkpoint systems to prevent T cells from attacking them. Researchers have developed antibody-based therapies targeting three of these systems:
- CTLA-4: The immune checkpoint protein CTLA-4 binds to CD80 and CD86 on antigen-presenting cells, preventing them from stimulating T-cell activation. CTLA-4 was the first immune checkpoint to be targeted by an FDA-approved therapy, ipilimumab (Yervoy), in 2011. Another CTLA-4 inhibitor, tremelimumab (Imjudo), first gained approval in 2022.
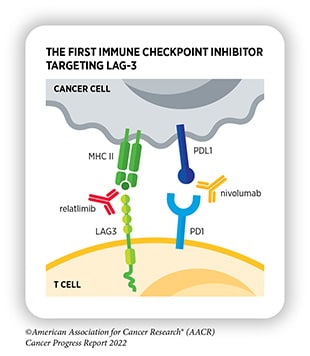
- PD-1/PD-L1: The immune checkpoint protein PD-1 binds to its ligand, PD-L1, on cancer cells, which sends a “don’t eat me” signal to the T cell, preventing it from attacking the tumor. Researchers have not only developed antibodies targeting PD-1 but those targeting PD-L1 as well. FDA-approved PD-1 inhibitors include pembrolizumab (Keytruda), first approved in 2014; nivolumab (Opdivo), first approved in 2014; and cemiplimab-rwlc (Libtayo), first approved in 2021. FDA-approved PD-L1 inhibitors include atezolizumab (Tecentriq), first approved in 2016; avelumab (Bavencio), first approved in 2017; and durvalumab (Imfinzi), first approved in 2017.
- LAG-3: The immune checkpoint protein LAG-3 has many ligands on tumor cells and antigen-presenting cells, and its activation helps to dampen T-cell activation. The first LAG-3 inhibitor, relatlimab-rmbw (used in combination with nivolumab under the brand name Opdualag), was approved in 2022.
Since 2011, these inhibitors have been approved to treat a wide range of cancer types, including cancers of the head and neck, breast, digestive tract, kidneys, skin, liver, lungs, blood, and female reproductive system. Certain ICIs are also approved to treat any solid tumor characterized by genetic vulnerabilities shown to increase the efficacy of ICI therapy. These vulnerabilities include high microsatellite instability, high tumor mutational burden, and DNA mismatch repair deficiencies.
As the indications for ICI therapy continue to expand, the number of patients who may benefit from ICI treatment grows. As with all therapies, however, the use of ICIs comes with some drawbacks. When T cells cannot be effectively regulated, they may attack healthy cells.
“Immune checkpoint inhibitors have been a huge revolution in cancer,” Salem said. “They wake up the immune system, but sometimes they wake up bad, autoreactive T cells that destroy your own body.”
Unfortunately, the heart is sometimes caught in the crossfire. Approximately 1% of cancer patients receiving ICI therapy develop myocarditis, which can cause pain, irregular heart rhythms, and sometimes heart failure.
While researchers aren’t entirely sure why T cells attack the heart, recent research suggests that the body may harbor T cells that target α-myosin, a protein abundantly expressed in the heart. Normally, the immune checkpoint ligands expressed on healthy cells prevent these autoreactive T cells from acting, but when the checkpoints are turned off, damage can occur.
Most skeletal muscles, including the thoracic muscles that support lung activity, also express the protein, leaving them vulnerable to attack. Salem explained that, in severe cases, this may cause a decline in respiratory function and the atrophy of muscles throughout the body, which can potentially be life-threatening.
These complications are rare, but as more cancer patients are treated with ICI therapy, the number of patients experiencing ICI-related muscle toxicity may increase. This necessitates the research, development, and testing of new treatment strategies for patients with ICI-related myocarditis and myotoxicity, Salem said.
Current Therapeutic Strategies
According to the National Comprehensive Cancer Network (NCCN) Guidelines, treatment for ICI-related myocarditis consists of ICI discontinuation, high-dose corticosteroids, and other immunomodulatory drugs, as necessary.
The first signs of cardiotoxicity may appear as an abnormal laboratory test, an arrhythmia seen on an echocardiogram (ECG), or an abnormality found on an imaging test. If such signs emerge, regardless of whether a patient shows symptoms, the NCCN Guidelines recommend permanent discontinuation of ICI treatment and a three- to five-day course of high-dose (1 gram/day) corticosteroids.
If symptoms improve, the patient can switch to 1 mg/kg/day of corticosteroids, a dose that must be tapered down over several weeks. If symptoms do not improve, physicians are encouraged to consider other immunomodulatory drugs, as well as supportive care to keep the heart beating.
Because of the potentially life-threatening nature of ICI-related heart damage, these interventions are proposed regardless of a patient’s clinical characteristics or the severity of the disease. Salem argued that the blanket use of high-dose corticosteroids could create additional hurdles to effective clinical management.
One potential problem is that T cells can rapidly develop resistance to corticosteroids. When this occurs, other immunomodulatory therapies must be employed, and they may not be as effective.
Another complication of high-dose corticosteroids is that tapering off must be done carefully. During corticosteroid treatment, the body will suppress the natural production of cortisol; if the external cortisol analogue is removed too quickly, the body may not produce enough to compensate. This can exacerbate the existing T-cell hyperactivity and worsen damage done by the immune system, Salem said.
Improving Patient Outcomes
Considering these complications, Salem argued that an approach that takes each patient’s symptoms and severity into account could be more beneficial than a one-size-fits-all treatment.
He and his colleagues further hypothesized that, instead of relying on corticosteroids that elicit widespread and nonspecific immunomodulatory effects, they might be able to treat ICI-related muscle toxicity by specifically targeting T-cell activation.

MD, PhD
Abatacept (Orencia) is a rheumatoid arthritis drug that is sometimes given to patients with ICI-related myocarditis when their disease progresses on corticosteroids. It binds to costimulatory receptors on antigen-presenting cells, which prevents them from stimulating T-cell activity. While abatacept can dampen a hyperactive immune response with long-lasting effects, it can take several weeks to reach optimal efficacy—time that some patients may not have.
Ruxolitinib—used to treat side effects, such as graft-versus-host disease, from other types of immunotherapy—inhibits the immune-stimulatory proteins JAK1 and JAK2, thereby also decreasing T-cell activation. While ruxolitinib has a short half-life and does not provide long-lasting effects, it begins working almost immediately, which Salem and colleagues hypothesized might help patients with severe disease while they wait for abatacept to kick in.
To test their ideas, they enrolled 40 consecutive cancer patients who were admitted to Assistance Publique–Hôpitaux de Paris with confirmed ICI-related myocarditis. They treated the first 10 according to the standard of care; the subsequent 30 were treated according to their disease severity and the extent of myotoxicity.
Of the 30 patients who received individualized care, four were diagnosed based on an abnormal lab test and did not have overt symptoms of myocarditis. Salem and colleagues discontinued ICI therapy in these patients but did not treat them with immune suppressants.
The other 26 patients were given low-dose corticosteroids and three infusions of high-dose (20 mg/kg) abatacept. The myocarditis was considered severe in 22 of these patients, and 17 of them were given ruxolitinib in addition to abatacept.
Salem and colleagues also considered it crucial to monitor all patients in the experimental treatment group for the spread of myotoxicity to the respiratory muscles, something Salem learned from treating patients in the standard of care group. Despite successful treatment of myocarditis, fatalities due to respiratory muscle failure can occur, he explained.
Ten patients in the experimental treatment group met the criteria for elective ventilation, and eight were placed on a ventilator.
Among patients in the standard-of-care group, the rate of myotoxicity-related mortality was 60 percent. In the experimental treatment group, however, the mortality rate was 3.3 percent; the one myotoxicity-related death in the experimental treatment group occurred in a patient who refused elective ventilation.
For patients in the standard-of-care group, the overall survival rate was 40 percent at three months and 20 percent at six months post-treatment. For patients in the experimental treatment group, the overall survival rate was 77 percent at three months and 70 percent at six months post-treatment.
“While this is not a randomized clinical trial, the significant improvement in outcomes when patients are treated with targeted therapies is very suggestive that this regimen is helpful,” Salem said.
He further emphasized that the personalized medicine aspect of this new regimen to manage myotoxicity is equally as important as the treatments themselves.
“We have to acknowledge that not all patients need the whole package; you may need all of it for the severe cases and only some of it for intermediate cases, or even none of it for persistently asymptomatic cases,” Salem said. “Even if it’s debated in the literature whether we should screen for and monitor the severity of every patient, for me, there’s no question.”



