From Bench to Bedside: New Frontiers in Multiple Myeloma
When teacher Ellen Reich began treatment for multiple myeloma in 2014, a sequence of therapies approved by the U.S. Food and Drug Administration (FDA) kept her cancer in check for over five years. Eventually, however, her disease returned, and she found herself short on options.

Reich’s doctor suggested that she enroll in a clinical trial testing CAR T-cell therapy in patients with multiple myeloma. The treatment involved harvesting T cells from Reich’s blood, editing the T cells to help them fight myeloma, expanding the edited T cells in the laboratory, and returning them to Reich’s bloodstream. Reich underwent the process in the summer of 2020.
The treatment removed all traces of multiple myeloma from Reich’s body. After weathering the failure of several prior treatments, Reich entered remission and has remained cancer-free to this day. Now retired, she volunteers with two multiple myeloma patient support organizations—a local group she established for patients in western Massachusetts and the HealthTree Foundation for Multiple Myeloma (formerly Myeloma Crowd), a national organization.
Today, the patients these groups support have more treatment options thanks to clinical trials like the one in which Reich participated. In the past two years, the FDA has approved three new immunotherapies—two types of CAR T therapy and a bispecific antibody—for the treatment of relapsed or refractory multiple myeloma.
In honor of Multiple Myeloma Awareness Month, we discuss how the latest clinical therapies are helping patients and how recent laboratory research is informing future treatments.
Fighting Immune Cells With Immune Cells
Blood cancers arise when white blood cells proliferate in an uncontrolled manner, resulting in too many cancerous cells and not enough healthy cells in the blood. In the case of multiple myeloma, the cancer forms from malignant plasma cells, a type of B cell that produces antibodies.
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute estimated that, in the year 2022, 34,470 individuals in the U.S. were diagnosed with multiple myeloma, and 12,640 Americans died from the disease. Between 2012 and 2018, patients with multiple myeloma had a relative five-year survival rate of 57.9 percent.
For many years, the standard first-line therapy for multiple myeloma has been high-dose chemotherapy plus a transplant of a patient’s own stem cells. Patients whose myeloma did not respond to or relapsed following a stem cell transplant and patients who are ineligible for a stem cell transplant commonly receive targeted therapies; these include proteasome inhibitors, histone deacetylase inhibitors, and nuclear export inhibitors. Other possible treatments include monoclonal antibodies targeting CD38 or SLAMF7 as well as the drugs thalidomide (Thalomid), lenalidomide (Revlimid), and pomalidomide (Pomalyst), which elicit nonspecific effects on the immune system.
Unfortunately, many patients develop resistance to these therapies, and the prognosis for these patients is often poor. As CAR T-cell therapies showed promising results in other relapsed or treatment-refractory blood cancers, they were eventually tested in patients with multiple myeloma.
In 2021, the FDA approved the CAR T-cell therapy idecabtagene vicleucel (ide-cel, Abecma) for the treatment of adult patients with multiple myeloma whose tumors did not respond to or relapsed following four or more prior lines of therapy. Ide-cel targets the protein BCMA, present on the surface of healthy and malignant plasma cells. When BCMA binds to the engineered T cells, it activates them, helping them to destroy the targeted cell.
In the phase II KarMMa clinical trial that secured the approval of ide-cel for patients with multiple myeloma, the overall response rate was 72%, and the complete response rate was 28%. In around 65% of patients with a complete response, the complete response persisted for 12 or more months.
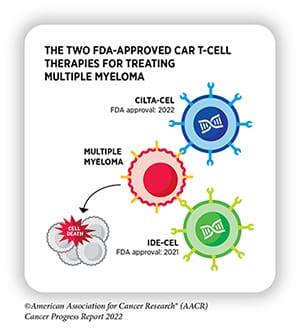
Less than a year later, the FDA approved ciltacabtagene autoleucel (cilta-cel, Carvykti), another BCMA-targeting CAR T-cell therapy, for the same indication. Over 97% of patients in the CARTITUDE-1 trial, on which the approval was based, experienced a response to cilta-cel, and the response lasted for a median of 21.8 months.
In addition to CAR T, researchers focused on developing a bispecific antibody, a two-pronged molecule that binds to both T cells and myeloma cells, bringing them together to help the former fight the latter. In October 2022, the FDA granted accelerated approval to teclistamab-cqyv (Tecvayli), the first bispecific antibody targeting BCMA. Like the CAR T-cell therapies, teclistamab was also approved to treat patients whose multiple myeloma was refractory to or relapsed following four or more lines of prior therapy.
In the phase II MajesTEC-1 clinical trial, consisting of 110 multiple myeloma patients who had received three or more prior therapies, the overall response rate was 61.8% after a median follow-up of 7.4 months.
New Discoveries Suggest New Treatments
No cancer therapy would make it to the market without years of basic, preclinical, and clinical research leading scientists to new drug targets and improved therapeutic strategies. Such discoveries have formed the backbone for the newest multiple myeloma treatments, and they continue to inform approaches that may help patients in the future.
The editors of Blood Cancer Discovery, a journal of the American Association for Cancer Research (AACR), have compiled a collection of basic and translational studies that have driven the field of multiple myeloma forward. These discoveries cover a broad range of topics at stages from clinical trials to mechanisms of myeloma cell signaling. A few of these topics are highlighted below:
New Treatments: While CAR T-cell therapies are currently approved for patients with multiple myeloma that progressed on or following other therapies, researchers are evaluating the efficacy of using CAR T cells to improve or extend an existing therapeutic response. In this phase I clinical trial, the researchers treated multiple myeloma patients with BCMA-targeting CAR T cells or a mixture of BCMA- and CD19-targeting CAR T cells. Two thirds of the patients with measurable disease experienced a partial response. Among patients still responding to their prior course of therapy, 34.6% improved their response category, including four who converted to a complete response.

Improving Treatment Delivery: Two articles in the collection—a perspective and a Science in Society piece—advocated for clinicians to evaluate how a patient’s background may influence the care they receive. In the perspective, researchers presented criteria to identify patients with high-risk multiple myeloma. They argued in favor of improved disease surveillance and specialized clinical trials for patients with high-risk disease in order to best tailor treatment strategies to their specific needs. The Science in Society article summarized recommendations made during a workshop hosted by the FDA and the AACR with the goal of increasing enrollment of African American patients into multiple myeloma clinical trials. The article included guidelines for increasing the diversity of enrollees into preapproval clinical trials and ensuring diversity-conscious analysis of data in postapproval clinical trials and analyses of real-world data.
Biomarkers of Prognosis: While CAR T-cell therapy benefits many patients with multiple myeloma, not all patients experience a response, necessitating new biomarkers that can help physicians determine who should receive the treatment. In one article, researchers reviewed some of the known determinants of CAR T response versus relapse after CAR T therapy. Some features that predict a poor response included changes in target expression, relatively fewer memory and CD4+ T cells in the CAR T product, more prior treatments, high cancer cell expression of PD-L1, and a tumor microenvironment inhabited by immune suppressive cells.

Understanding Myeloma Cells: New drug targets are often based on the knowledge of how cancer cells function on a molecular level. Several articles in this collection described new insights that may lead to new therapies for multiple myeloma. In one article, researchers identified lysine demethylase 5A as a crucial mediator of oncogenic MYC signaling in myeloma cells. A different group of researchers showed that the proteasome inhibitor bortezomib (Velcade) helps to boost antitumor immune responses against multiple myeloma, suggesting it may form an effective combination with some immunotherapies. Another study showed that BCMA-targeting bispecific antibodies were less effective in patients with a high tumor burden and that cotreatment with cyclophosphamide helped overcome this difference.
For more information about these studies, as well as other recent advances in multiple myeloma research, check out the full collection here. Reich shared her story with Cancer Today, the AACR’s online resource and magazine for cancer patients, survivors, and their family members and friends. The print edition of Cancer Today is free to subscribers who live in the U.S. Subscribe here to receive four issues per year.



