AACR Journals Share Selected Articles for March
As we welcome spring, enjoy the latest round of editors’ picks selected by the editors of AACR’s 10 journals. Among this month’s highlights are an analysis of the racial/ethnic and sex-related differences in genomic mutations among patients with early-onset colorectal cancer; a study on the association of nonsteroidal anti-inflammatory agents and breast cancer risk among patients with benign breast disease; a phase I trial evaluating mistletoe extract in advanced cancer; and more.
The abstracts of these studies are included below, and the full text of each article will be freely available for a limited time.
Journal: Blood Cancer Discovery
Low hypodiploidy defines a rare subtype of B-cell acute lymphoblastic leukemia (B-ALL) with a dismal outcome. To investigate the genomic basis of low-hypodiploid ALL (LH-ALL) in adults, we analyzed copy-number aberrations, loss of heterozygosity, mutations, and cytogenetics data in a prospective cohort of Philadelphia (Ph)-negative B-ALL patients (n = 591, ages 18–84 years), allowing us to identify 80 LH-ALL cases (14%). Genomic analysis was critical for evidencing low hypodiploidy in many cases missed by cytogenetics. The proportion of LH-ALL within Ph-negative B-ALL dramatically increased with age, from 3% in the youngest patients (under 40 years old) to 32% in the oldest (over 55 years old). Somatic TP53 biallelic inactivation was the hallmark of adult LH-ALL, present in virtually all cases (98%). Strikingly, we detected TP53 mutations in posttreatment remission samples in 34% of patients. Single-cell proteogenomics of diagnosis and remission bone marrow samples evidenced a preleukemic, multilineage, TP53-mutant clone, reminiscent of age-related clonal hematopoiesis. Significance: We show that low-hypodiploid ALL is a frequent entity within B-ALL in older adults, relying on somatic TP53 biallelic alteration. Our study unveils a link between aging and low-hypodiploid ALL, with TP53-mutant clonal hematopoiesis representing a preleukemic reservoir that can give rise to aneuploidy and B-ALL.
This article was highlighted in the March issue, and a related commentary is available here.
Journal: Cancer Discovery
Molecular features underlying colorectal cancer disparities remain uncharacterized. Here, we investigated somatic mutation patterns by race/ethnicity and sex among 5,856 non-Hispanic white (NHW), 535 non-Hispanic Black (NHB), and 512 Asian/Pacific Islander (API) patients with colorectal cancer (2,016 early-onset colorectal cancer patients: sequencing age <50 years). NHB patients with early-onset nonhypermutated colorectal cancer, but not API patients, had higher adjusted tumor mutation rates than NHW patients. There were significant differences for LRP1B, FLT4, FBXW7, RNF43, ATRX, APC, and PIK3CA mutation frequencies in early-onset nonhypermutated colorectal cancers between racial/ethnic groups. Heterogeneities by race/ethnicity were observed for the effect of APC, FLT4, and FAT1 between early-onset and late-onset nonhypermutated colorectal cancer. By sex, heterogeneity was observed for the effect of EP300, BRAF, WRN, KRAS, AXIN2, and SMAD2. Males and females with nonhypermutated colorectal cancer had different trends in EP300 mutations by age group. These findings define genomic patterns of early-onset nonhypermutated colorectal cancer by race/ethnicity and sex, which yields novel biological clues into early-onset colorectal cancer disparities. Significance: NHBs, but not APIs, with early-onset nonhypermutated colorectal cancer had higher adjusted tumor mutation rates versus NHWs. Differences for FLT4, FBXW7, RNF43, LRP1B, APC, PIK3CA, and ATRX mutation rates between racial/ethnic groups and EP300, KRAS, AXIN2, WRN, BRAF, and LRP1B mutation rates by sex were observed in tumors of young patients.
This study was featured on the cover and highlighted in the March issue. A related commentary is available here.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: While cigarette smoking is the leading cause of lung cancer, the majority of smokers do not develop the disease over their lifetime. The inter-individual differences in risk among smokers may in part be due to variations in exposure to smoking-related toxicants. Methods: Using data from a subcohort of 2,309 current smokers at the time of urine collection from the Multiethnic Cohort Study, we prospectively evaluated the association of ten urinary biomarkers of smoking-related toxicants [total nicotine equivalents (TNE), a ratio of total trans-3′-hydroxycotinine (3-HCOT)/cotinine (a phenotypic measure of CYP2A6 enzymatic activity), 4-(methylnitrosamino)-1–3-(pyridyl)-1-butanol (NNAL), S-phenylmercapturic acid (SPMA), 3-hydroxypropyl mercapturic acid (3-HPMA), phenanthrene tetraol (PheT), 3-hydroxyphenanthrene (PheOH), the ratio of PheT/PheOH, cadmium (Cd), and (Z)-7-(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopenyl]hept-5-enoic acid (8-iso-PGF2α)] with lung cancer risk (n = 140 incident lung cancer cases over an average of 13.4 years of follow-up). Lung cancer risk was estimated using Cox proportional hazards models. Results: After adjusting for decade of birth, sex, race/ethnicity, body mass index, self-reported pack-years, creatinine, and urinary TNE (a biomarker of internal smoking dose), a one SD increase in log total 3-HCOT/cotinine (HR, 1.33; 95% CI, 1.06–1.66), 3-HPMA (HR, 1.41; 95% CI, 1.07–1.85), and Cd (HR, 1.45; 95% CI, 1.18–1.79) were each associated with increased lung cancer risk. Conclusions: Our study demonstrates that urinary total 3-HCOT/cotinine, 3-HPMA, and Cd are positively associated with lung cancer risk. These findings warrant replication and consideration as potential biomarkers for smoking-related lung cancer risk. Impact: These biomarkers may provide additional information on lung cancer risk that is not captured by self-reported smoking history or TNE.
This article was highlighted in the March issue, and a related commentary is available here.
Journal: Cancer Immunology Research
Syntaphilin Regulates Neutrophil Migration in Cancer
Pathologically activated neutrophils (PMN) with immunosuppressive activity, which are termed myeloid-derived suppressor cells (PMN-MDSC), play a critical role in regulating tumor progression. These cells have been implicated in promoting tumor metastases by contributing to premetastatic niche formation. This effect was facilitated by enhanced spontaneous migration of PMN from bone marrow to the premetastatic niches during the early stage of cancer development. The molecular mechanisms underpinning this phenomenon remained unclear. In this study, we found that syntaphilin (SNPH), a cytoskeletal protein previously known for anchoring mitochondria to the microtubule in neurons and tumor cells, could regulate migration of PMN. Expression of SNPH was decreased in PMN from tumor-bearing mice and patients with cancer as compared with PMN from tumor-free mice and healthy donors, respectively. In Snph-knockout (SNPH-KO) mice, spontaneous migration of PMN was increased and the mice showed increased metastasis. Mechanistically, in SNPH-KO mice, the speed and distance travelled by mitochondria in PMN was increased, rates of oxidative phosphorylation and glycolysis were elevated, and generation of adenosine was increased. Thus, our study reveals a molecular mechanism regulating increased migratory activity of PMN during cancer progression and suggests a novel therapeutic targeting opportunity.
Journal: Cancer Prevention Research
Benign Breast Disease, NSAIDs, and Postmenopausal Breast Cancer Risk in the CPS-II Cohort
Nonsteroidal anti-inflammatory agents (NSAID) are associated with modest inconsistent reductions in breast cancer risk in population-based cohorts, whereas two focused studies of patients with benign breast disease (BBD) have found lower risk with NSAID use. Given that BBD includes fibroinflammatory lesions linked to elevated breast cancer risk, we assessed whether NSAID use was associated with lower breast cancer risk among patients with BBD. Participants were postmenopausal women in the Cancer Prevention Study-II (CPS-II), a prospective study of cancer incidence and mortality, who completed follow-up surveys in 1997 with follow-up through June 30, 2015. History of BBD, NSAID use, and covariate data were updated biennially. This analysis included 23,615 patients with BBD and 36,751 patients with non-BBD, including 3,896 incident breast cancers over an average of 12.72 years of follow-up among participants. NSAID use, overall and by formulation, recency, duration, and pills per month was analyzed versus breast cancer risk overall and by BBD status using multivariable-adjusted Cox models; BBD status and NSAID use were modeled as time-dependent exposures. Patients with BBD who reported using NSAIDs experienced lower breast cancer risk (HR, 0.87; 95% CI, 0.78–0.97), with similar effects for estrogen receptor (ER)-positive breast cancers [HR, 0.85; 95% confidence interval (CI), 0.74–0.97] and ER-negative breast cancers (HR, 0.87; 95% CI, 0.59–1.29); among women without BBD, NSAID use was unrelated to risk (HR, 1.02; 95% CI, 0.92–1.13; Pinteraction = 0.04). Associations stratified by age, obesity, menopausal hormone use, and cardiovascular disease were similar. Among patients with BBD, NSAID use appears linked to lower breast cancer risk. Further studies to assess the value of NSAID use among patients with BBD are warranted. Prevention Relevance: We examined whether NSAID use, a modifiable exposure, is associated with breast cancer risk in postmenopausal women from the Cancer Prevention Study-II with self-reported benign breast disease, an often inflammatory condition associated with higher rates of breast cancer.
Journal: Cancer Research (March 1 issue)
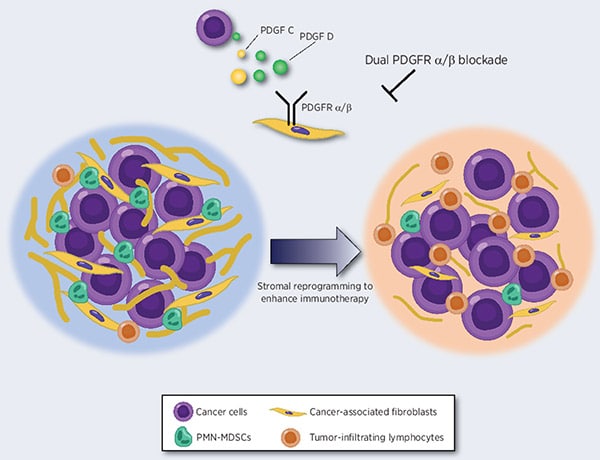
Excess stroma and cancer-associated fibroblasts (CAF) enhance cancer progression and facilitate immune evasion. Insights into the mechanisms by which the stroma manipulates the immune microenvironment could help improve cancer treatment. Here, we aimed to elucidate potential approaches for stromal reprogramming and improved cancer immunotherapy. Platelet-derived growth factor C (PDGFC) and D expression were significantly associated with a poor prognosis in patients with gastric cancer, and PDGF receptor beta (PDGFRβ) was predominantly expressed in diffuse-type gastric cancer stroma. CAFs stimulated with PDGFs exhibited markedly increased expression of CXCL1, CXCL3, CXCL5, and CXCL8, which are involved in polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC) recruitment. Fibrotic gastric cancer xenograft tumors exhibited increased PMN-MDSC accumulation and decreased lymphocyte infiltration, as well as resistance to anti–PD-1. Single-cell RNA sequencing and spatial transcriptomics revealed that PDGFRα/β blockade reversed the immunosuppressive microenvironment through stromal modification. Finally, combining PDGFRα/β blockade and anti–PD-1 treatment synergistically suppressed the growth of fibrotic tumors. These findings highlight the impact of stromal reprogramming on immune reactivation and the potential for combined immunotherapy for patients with fibrotic cancer. Significance: Stromal targeting with PDGFRα/β dual blockade reverses the immunosuppressive microenvironment and enhances the efficacy of immune checkpoint inhibitors in fibrotic cancer.
A commentary related to this study is available here.
Journal: Cancer Research (March 15 issue)
Malignant gliomas such as glioblastoma are highly heterogeneous with distinct cells of origin and varied genetic alterations. It remains elusive whether the specific states of neural cell lineages are differentially susceptible to distinct genetic alterations during malignant transformation. Here, an analysis of The Cancer Genome Atlas databases revealed that comutations of PTEN and TP53 are most significantly enriched in human high-grade gliomas. Therefore, we selectively ablated Pten and Trp53 in different progenitors to determine which cell lineage states are susceptible to malignant transformation. Mice with PTEN/p53 ablation mediated by multilineage-expressing human GFAP (hGFAP) promoter–driven Cre developed glioma but with incomplete penetrance and long latency. Unexpectedly, ablation of Pten and Trp53 in Nestin+ neural stem cells (NSC) or Pdgfra+/NG2+ committed oligodendrocyte precursor cells (OPC), two major cells of origin in glioma, did not induce glioma formation in mice. Strikingly, mice lacking Pten and Trp53 in Olig1+/Olig2+ intermediate precursors (pri-OPC) prior to the committed OPCs developed high-grade gliomas with 100% penetrance and short latency. The resulting tumors exhibited distinct tumor phenotypes and drug sensitivities from NSC- or OPC-derived glioma subtypes. Integrated transcriptomic and epigenomic analyses revealed that PTEN/p53-loss induced activation of oncogenic pathways, including HIPPO-YAP and PI3K signaling, to promote malignant transformation. Targeting the core regulatory circuitries YAP and PI3K signaling effectively inhibited tumor cell growth. Thus, our multicell state in vivo mutagenesis analyses suggests that transit-amplifying states of Olig1/2 intermediate lineage precursors are predisposed to PTEN/p53-loss–induced transformation and gliomagenesis, pointing to subtype-specific treatment strategies for gliomas with distinct genetic alterations. Significance: Multiple progenitor-state mutagenesis reveal that Olig1/2-expressing intermediate precursors are highly susceptible to PTEN/p53-loss–mediated transformation and impart differential drug sensitivity, indicating tumor-initiating cell states and genetic drivers dictate glioma phenotypes and drug responses.
A commentary related to this study is available here.
Journal: Clinical Cancer Research (February 1 issue)
Purpose: Preclinical data suggest that antiprogestins inhibit the growth of luminal breast carcinomas that express higher levels of progesterone receptor isoform A (PRA) than isoform B (PRB). Thus, we designed a presurgical window of opportunity trial to determine the therapeutic effects of mifepristone in patients with breast cancer, based on their high PRA/PRB isoform ratio (MIPRA; NCT02651844). Patients and Methods: Twenty patients with luminal breast carcinomas with PRA/PRB > 1.5 (determined by Western blots), and PR ≥ 50%, naïve from previous treatment, were included for mifepristone treatment (200 mg/day orally; 14 days). Core needle biopsies and surgical samples were formalin fixed for IHC studies, while others were snap-frozen to perform RNA sequencing (RNA-seq), proteomics, and/or Western blot studies. Plasma mifepristone levels were determined using mass spectrometry. The primary endpoint was the comparison of Ki67 expression pretreatment and posttreatment. Results: A 49.62% decrease in Ki67 staining was observed in all surgical specimens compared with baseline (P = 0.0003). Using the prespecified response parameter (30% relative reduction), we identified 14 of 20 responders. Mifepristone induced an increase in tumor-infiltrating lymphocytes; a decrease in hormone receptor and pSer118ER expression; and an increase in calregulin, p21, p15, and activated caspase 3 expression. RNA-seq and proteomic studies identified downregulated pathways related to cell proliferation and upregulated pathways related to immune bioprocesses and extracellular matrix remodeling. Conclusions: Our results support the use of mifepristone in patients with luminal breast cancer with high PRA/PRB ratios. The combined effects of mifepristone and estrogen receptor modulators warrant clinical evaluation to improve endocrine treatment responsiveness in these patients.
This article was highlighted in the March issue, and a related commentary is available here.
Journal: Clinical Cancer Research (February 15 issue)
Purpose: BRAF V600E mutant metastatic colorectal cancer represents a significant clinical problem, with combination approaches being developed clinically with oral BRAF inhibitors combined with EGFR-targeting antibodies. While compelling preclinical data have highlighted the effectiveness of combination therapy with vemurafenib and small-molecule EGFR inhibitors, gefitinib or erlotinib, in colorectal cancer, this therapeutic strategy has not been investigated in clinical studies. Patients and Methods: We conducted a phase Ib/II dose-escalation/expansion trial investigating the safety/efficacy of the BRAF inhibitor vemurafenib and EGFR inhibitor erlotinib. Results: Thirty-two patients with BRAF V600E positive metastatic colorectal cancer (mCRC) and 7 patients with other cancers were enrolled. No dose-limiting toxicities were observed in escalation, with vemurafenib 960 mg twice daily with erlotinib 150 mg daily selected as the recommended phase II dose. Among 31 evaluable patients with mCRC and 7 with other cancers, overall response rates were 32% [10/31, 16% (5/31) confirmed] and 43% (3/7), respectively, with clinical benefit rates of 65% and 100%. Early ctDNA dynamics were predictive of treatment efficacy, and serial ctDNA monitoring revealed distinct patterns of convergent genomic evolution associated with acquired treatment resistance, with frequent emergence of MAPK pathway alterations, including polyclonal KRAS, NRAS, and MAP2K1 mutations, and MET amplification. Conclusions: The Erlotinib and Vemurafenib In Combination Trial study demonstrated a safe and novel combination of two oral inhibitors targeting BRAF and EGFR. The dynamic assessment of serial ctDNA was a useful measure of underlying genomic changes in response to this combination and in understanding potential mechanisms of resistance.
This article was highlighted in the March 15 issue.
Journal: Molecular Cancer Research
Ataxia-telangiectasia mutated (ATM) is an apical regulator of responses to DNA double-strand breaks (DSB). Using two complementary unbiased proteomic screens, we identified the cohesin complex proteins PDS5A, PDS5B, RAD21, NIPBL, and WAPL as apparent novel ATM interactors and substrates. ATM-dependent phosphorylation of PDS5A on Ser1278 following treatment with ionizing radiation is required for optimal cell survival, cell-cycle checkpoint activation, and chromosomal stability. Using a system that introduces site-specific DNA breaks, we found that ATM phosphorylation of cohesin proteins SMC1A, SMC3, and PDS5A are all required for repression of both RNA transcription and DNA replication within the vicinity of a DSB, the latter insight based on development of a novel localized S-phase cell-cycle checkpoint assay. These findings highlight the significance of interactions between ATM and cohesin in the regulation of DNA metabolic processes by altering the chromatin environment surrounding a DSB. Implications: Multiple members of the cohesin complex are involved in the regulation of DNA replication and transcription in the vicinity of DNA double-strand breaks and their role(s) are regulated by the ATM kinase.
This article was highlighted in this issue.
Journal: Molecular Cancer Therapeutics
Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem
Studying the complex mechanisms of tumorigenesis and examining the interactions of neoplastic cells within tumor ecosystem are critical to explore the possibility of effective cancer treatment modalities. Dynamic tumor ecosystem is constantly evolving and is composed of tumor cells, extracellular matrix (ECM), secreted factors, and stromal cancer-associated fibroblasts (CAF), pericytes, endothelial cells (EC), adipocytes, and immune cells. ECM remodeling by synthesis, contraction, and/or proteolytic degradation of ECM components and release of matrix-sequestered growth factors create a microenvironment that promotes EC proliferation, migration, and angiogenesis. Stromal CAFs release multiple angiogenic cues (angiogenic growth factors, cytokines, and proteolytic enzymes) which interact with ECM proteins, thus contribute to enhance proangiogenic/promigratory properties and support aggressive tumor growth. Targeting angiogenesis brings about vascular changes including reduced adherence junction proteins, basement membrane and pericyte coverage, and increased leakiness. This facilitates ECM remodeling, metastatic colonization and chemoresistance. Owing to significant role of denser and stiffer ECM in inducing chemoresistance, direct or indirect targeting of ECM components is being reported as major axis of anticancer treatment. Exploring the agents targeting angiogenesis and ECM in a context specific manner may lead to reduced tumor burden by promoting conventional therapeutic effectiveness and overcoming the hurdles of therapy resistance.
Journal: Cancer Research Communications
Phase I Trial of Intravenous Mistletoe Extract in Advanced Cancer
Purpose: Mistletoe extract (ME) is widely used for patients with cancer to support therapy and to improve quality of life (QoL). However, its use is controversial due to suboptimal trials and a lack of data supporting its intravenous administration. Materials and Methods: This phase I trial of intravenous mistletoe (Helixor M) aimed to determine the recommended phase II dosing and to evaluate safety. Patients with solid tumor progressing on at least one line of chemotherapy received escalating doses of Helixor M three times a week. Assessments were also made of tumor marker kinetics and QoL. Results: Twenty-one patients were recruited. The median follow-up duration was 15.3 weeks. The MTD was 600 mg. Treatment-related adverse events (AE) occurred in 13 patients (61.9%), with the most common being fatigue (28.6%), nausea (9.5%), and chills (9.5%). Grade 3+ treatment-related AEs were noted in 3 patients (14.8%). Stable disease was observed in 5 patients who had one to six prior therapies. Reductions in baseline target lesions were observed in 3 patients who had two to six prior therapies. Objective responses were not observed. The disease control rate (percentage of complete/partial response and stable disease) was 23.8%. The median stable disease was 15 weeks. Serum cancer antigen-125 or carcinoembryonic antigen showed a slower rate of increase at higher dose levels. The median QoL by Functional Assessment of Cancer Therapy-General increased from 79.7 at week 1 to 93 at week 4. Conclusions: Intravenous mistletoe demonstrated manageable toxicities with disease control and improved QoL in a heavily pretreated solid tumor population. Future phase II trials are warranted. Significance: Although ME is widely used for cancers, its efficacy and safety are uncertain. This first phase I trial of intravenous mistletoe (Helixor M) aimed to determine phase II dosing and to evaluate safety. We recruited 21 patients with relapsed/refractory metastatic solid tumor. Intravenous mistletoe (600 mg, 3/week) demonstrated manageable toxicities (fatigue, nausea, and chills) with disease control and improved QoL. Future research can examine ME’s effect on survival and chemotherapy tolerability.



