Parallel Approaches Reveal the Microenvironment’s Role in Pancreatic Cancer Onset and Spread
The milieu of cells, proteins, and vasculature that comprise the tumor microenvironment can hinder efforts to treat cancer. This is especially true in pancreatic cancer, where a dense and immunologically inert tumor microenvironment prevents drugs from reaching cancer cells and stifles antitumor immune responses.
How the pancreatic tumor microenvironment develops, however, remains unclear. Understanding this process will be key to developing effective early detection, interception, and therapeutic approaches for this highly lethal disease.
To this end, researcher Marina Pasca di Magliano, PhD, is undertaking several parallel approaches to define changes to the microenvironment throughout disease progression, from normal pancreatic tissue to precancer, invasive cancer, and metastatic cancer.
One approach, which was recently published in the AACR journal Cancer Discovery, relied on a partnership with Gift of Life Michigan to analyze difficult-to-obtain normal pancreatic tissue, an important comparator when studying changes associated with cancer.
“Unfortunately, it has been difficult to understand the baseline characteristics of the pancreas due to a lack of normal pancreatic tissue available for research,” said Pasca di Magliano. Her co-author Timothy Frankel, MD, explained that in lieu of normal pancreatic tissue, researchers have historically relied on the tissue surrounding pancreatic tumors as a substitute for normal tissue.
“However, it is clear that the tissue adjacent to tumors is very abnormal looking and is not a reliable surrogate for true, healthy pancreatic tissue,” he said.
To characterize normal pancreatic tissue, Pasca di Magliano, Frankel, and colleagues, including first author Eileen Carpenter, MD, PhD, obtained healthy pancreata from 30 recently deceased donors for whom no suitable transplant recipients were identified. Because the pancreata were donated following brain death, blood flow was maintained until the organ could be surgically removed and immediately cooled, which helped preserve the tissue.
Analyses of these tissues revealed the presence of a cancer precursor, pancreatic intraepithelial neoplasia (PanIN), in over half of the donated pancreata across several age and racial groups, suggesting that these precancerous lesions may occur more frequently than previously thought.
“Given that pancreatic cancer is exceedingly rare, the widespread occurrence of PanINs in individuals of various age and race challenges the paradigm that PanINs always evolve into cancer,” said Carpenter.
“Understanding why some PanINs evolve to cancer and others do not will be important to accurately predict who is at risk of pancreatic cancer and to develop techniques for cancer interception,” Pasca di Magliano said. “The composition of the microenvironments surrounding PanINs might be a key factor.”
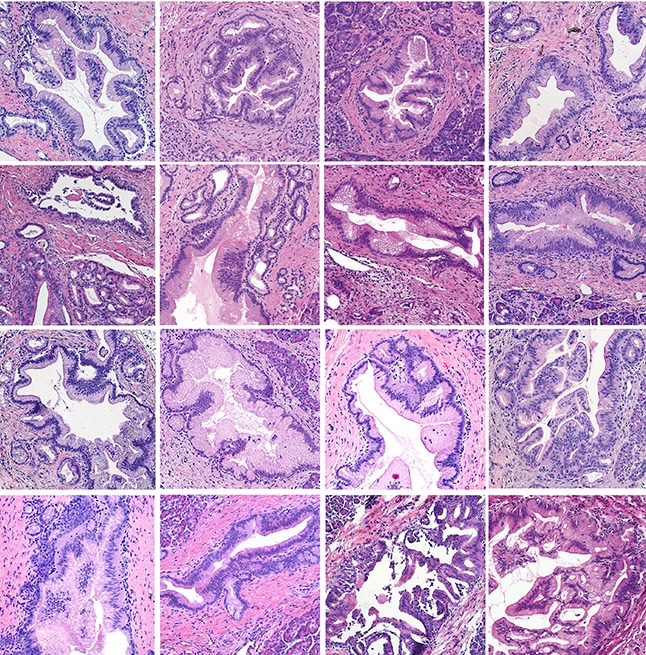
The microenvironments surrounding PanIN lesions were found to be distinct from those of both normal and cancerous pancreatic tissue; however, gene expression patterns were similar between PanINs and pancreatic tumors. Like 90% of pancreatic cancers, PanINs frequently harbored oncogenic mutations in the KRAS gene.
At the AACR Special Conference: Targeting RAS, held March 5-8, Pasca di Magliano reported that oncogenic mutation of KRAS in pancreatic epithelial cells led to PanIN formation and establishment of the PanIN microenvironment in mice, including the activation of surrounding fibroblasts through pro-tumor gene expression changes. Oncogenic KRAS was also required for PanIN maintenance and progression to invasive pancreatic cancer.
In addition to modulating the local pancreatic microenvironment, oncogenic KRAS in pancreatic epithelial cells promoted fibroblast activation and expansion in the lungs, thereby priming the mouse lungs for colonization by metastatic pancreatic cancer cells. In the future, Pasca di Magliano and colleagues plan to analyze lung tissue from deceased donors to determine if their observations hold true in humans.
Together, the results suggest that PanINs may be common in the adult population and may have a distinct microenvironment driven by oncogenic KRAS. Furthermore, they suggest that oncogenic KRAS’s effects on the microenvironment may influence many stages of pancreatic cancer—from PanIN formation to progression to invasive cancer to distant metastasis.