FDA Approvals in Oncology: April-June 2023
With the approval of new anticancer therapeutics, more treatment options become available for patients. Some therapies are new to the market, while some may have already been approved for other indications; some molecules are first in class, directed against a previously untargeted pathway or acting through a new mechanism, while others may be improved versions of drugs that already exist.
To help our readers keep track of the cancer therapies approved by the U.S. Food and Drug Administration (FDA), understand their impact for patients, and put them in context of the current therapeutic landscape, Cancer Research Catalyst provides a quarterly review of the latest from the FDA.
Approvals issued from April to June 2023 included antibody-drug conjugates for multiple cancer types, bispecific T-cell engagers for B-cell malignancies, a modified stem cell therapy based on umbilical cord blood, and two PARP inhibitors for the treatment of certain prostate cancers.
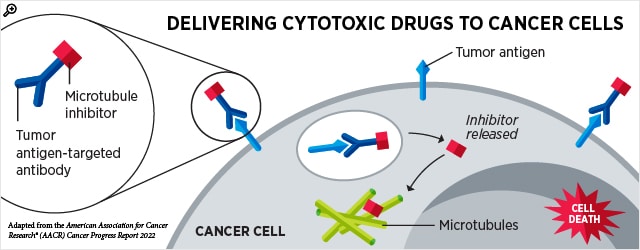
Using antibodies to home in on cancer cells
Traditional cancer treatments, such as chemotherapy and radiation therapy, kill cancerous and noncancerous cells alike, which can lead to debilitating side effects in patients. To mitigate these side effects, newer cancer therapies aim to selectively kill cancer cells while sparing healthy cells.
A strategy to home in on cancer cells is to utilize antibodies, which can be engineered to recognize proteins that are primarily found on cancer cells. One class of therapy, known as an antibody-drug conjugate, consists of a toxic drug linked to an antibody. When the antibody binds to the target protein on the cancer cell, the antibody-drug conjugate is internalized, and the toxic drug is released into the cell where it causes cell death. This design allows the drug to be delivered only to cells that express the target protein, which, in most cases, would be cancer cells.
Several antibody-drug conjugates have been approved by the FDA for a variety of cancer types. Over the past three months, the FDA approved two more, one for bladder cancer and another for certain hematologic cancers.
- In April, enfortumab vedotin-ejfv (Padcev) was approved in combination with pembrolizumab (Keytruda) as a first-line treatment for locally advanced or metastatic bladder cancer. First described in a 2016 report in the AACR journal Cancer Research, enfortumab vedotin-ejfv is comprised of an antibody that binds the nectin-4 protein found on many bladder cancer cells and a cell-killing drug called MMAE.
Enfortumab vedotin-ejfv was previously approved as a monotherapy for patients with locally advanced or metastatic bladder cancer who had received prior treatment. The latest approval expands the use of the drug to patients who have not received any treatment, many of whom are ineligible for first-line standard-of-care chemotherapy, and combines it with immunotherapy. Research presented at the AACR Annual Meeting 2020 indicated that enfortumab vedotin-ejfv induces the release of immune-stimulating molecules from dying cells; therefore, combining the therapy with pembrolizumab may augment the antitumor immune response.
- Also in April, polatuzumab vedotin-piiq (Polivy) was approved in combination with a steroid and chemotherapy regimen (commonly referred to as R-CHP) as first-line treatment for certain patients with diffuse large B-cell lymphoma, not otherwise specified (DLBCL-NOS), or high-grade B-cell lymphoma (HGBCL). Polatuzumab vedotin-piiq binds the CD79b protein that is expressed on the surface of malignant B cells; internalization of the antibody-drug conjugate releases MMAE into the cells, leading to cell death.
Standard first-line treatment for patients with these cancers is a steroid and chemotherapy regimen that includes the chemotherapeutic vincristine (this regimen is referred to as R-CHOP); however, only 60% of patients experience lasting responses. The approval of polatuzumab vedotin-piiq plus R-CHP provides a more effective treatment option for this patient population. In a clinical trial, patients who received first-line polatuzumab vedotin-piiq plus R-CHP were significantly less likely to experience disease progression than patients who received the standard R-CHOP therapy.
In addition to directing toxic drugs to cancer cells, antibodies may also be used to guide cytotoxic immune cells to cancer cells. Bispecific T cell engagers (BiTEs) are antibodies that simultaneously bind two proteins—one on cancer cells and the other on T cells—forming a bridge to bring these cells in proximity to one another.
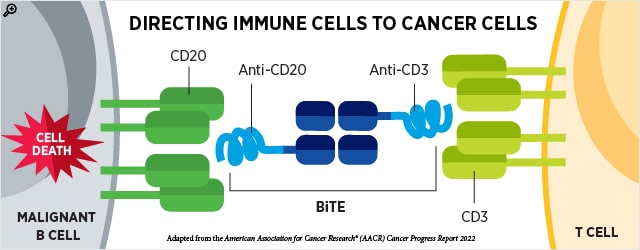
This quarter, the FDA granted accelerated approval to two BiTEs to treat patients with certain relapsed or refractory B-cell lymphomas. Accelerated approval means that continued approval may be contingent upon a confirmatory trial.
Patients with relapsed or refractory B-cell lymphomas have historically had limited treatment options. The advent of chimeric antigen receptor (CAR) T-cell therapy in recent years has provided another option; however, many patients do not have access to or sufficient time for this highly specialized and personalized therapy. The newly approved BiTEs offer effective off-the-shelf therapies for patients with certain B-cell lymphomas.
- In May, epcoritamab-bysp (Epkinly) received accelerated approval as a treatment for patients with relapsed or refractory DLBCL-NOS or HGBCL who have undergone at least two prior lines of systemic therapy. Epcoritamab-bysp is a BiTE that binds both the CD3 receptor on T cells and CD20 on malignant B cells. This is the first BiTE to receive FDA approval for DLBCL or HGBCL and adds to the growing list of BiTEs available for hematologic cancers. Earlier results from the clinical trial evaluating epcoritamab-bysp were described in the AACR journal Cancer Discovery.
- In June, glofitamab-gxbm (Columvi) received accelerated approval to treat patients with relapsed or refractory DLBCL-NOS or large B-cell lymphoma (LBCL) arising from follicular lymphoma who have received two or more prior lines of systemic therapy. Like epcoritamab-bysp, glofitamab-gxbm is a BiTE that binds the CD3 receptor on T cells and CD20 on malignant B cells. A study presented at the AACR Annual Meeting 2022 identified features associated with clinical response to glofitamab-gxbm.
This quarter, the FDA also granted full approval to a BiTE that had received an accelerated approval in 2018 to treat certain leukemias.
- In June, blinatumomab (Blincyto) received full approval to treat adult and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia (B-ALL) who are in first or second complete remission and have a certain level of minimal residual disease. Blinatumomab is a BiTE that binds CD19 on leukemia cells and the CD3 receptor on T cells.
Modifying umbilical cord blood-derived stem cells
Patients with hematologic malignancies may undergo stem cell transplantation to replace their cancerous blood cells with hematopoietic stem cells that will develop into healthy blood cells. Umbilical cord blood is an important source of hematopoietic stem cells for this procedure, but its use has been associated with fatal infections that arise before the healthy blood cells have repopulated and immune activity is restored.
A modified approach, which involves treating umbilical cord blood with nicotinamide prior to transplantation to increase the number of hematopoietic stem cells, may mitigate the risk of these infections and was approved by the FDA this quarter.
- The therapy, omidubicel-onlv (Omisirge), was approved for patients 12 years of age and older who are scheduled for myeloablative conditioning and subsequent umbilical cord blood transplantation as a treatment for their cancer. Compared to the standard approach, in which umbilical cord blood is not pretreated with nicotinamide, omidubicel-onlv led to faster recovery of neutrophils and was associated with a lower risk of certain infections.
Making PARP inhibition available earlier for patients with metastatic castration-resistant prostate cancer
Typical treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) includes androgen-blocking therapy or chemotherapy, but most patients survive less than three years with these treatments. Certain patients whose mCRPC progresses on androgen-blocking therapy may be treated with inhibitors of the PARP protein, which regulates activity of the androgen receptor and is involved in DNA repair.
This quarter, two approvals from the FDA made PARP inhibition available to patients together with androgen-blocking therapy, thereby moving PARP inhibition to an earlier line of treatment.
- In May, the FDA approved olaparib (Lynparza) in combination with abiraterone (Zytiga) and a corticosteroid for patients whose mCRPC harbors mutations in a BRCA gene.
- In June, the FDA approved talazoparib (Talzenna) in combination with enzalutamide (Xtandi) for patients whose mCRPC harbors mutations in a BRCA gene or in other genes involved in homologous recombination, a form of DNA repair.
Olaparib and talazoparib are approved to treat several BRCA-mutated cancers, where combined suppression of the DNA repair processes in which BRCA and PARP are involved is thought to cause the accumulation of DNA damage and ultimately cell death. In 2020, olaparib and another PARP inhibitor, rucaparib (Rubraca), received FDA approval to treat BRCA-mutated mCRPC that progresses after prior androgen-blocking therapy, such as abiraterone or enzalutamide.
The latest approvals are based on clinical trial data showing that adding olaparib or talazoparib to abiraterone or enzalutamide, respectively, lowered the risk of disease progression or death in patients with mCRPC harboring certain genetic mutations.
For more details, including the full indications and the clinical trial results that led to each approval, visit the FDA approvals page on the AACR website.