Urine Tests: Can Detecting Cancer Become Easy Pee-sy?
It’s noninvasive … it’s easy to collect … it’s urine tests! The advantages that come with being able to detect signs of cancer from urine led Philip Abbosh, MD, PhD, of Fox Chase Cancer Center, to lightheartedly compare this form of liquid biopsy to Superman during a session at the AACR Special Conference in Cancer Research: Bladder Cancer: Transforming the Field, held May 17-20.
“I think, as do many others, urine tests will transform the way we take care of patients clinically,” Abbosh said.
For example, Abbosh and his team are working on a urine test to detect residual bladder cancer following neoadjuvant therapy to predict which patients could avoid cystectomy, the partial or complete removal of the bladder. But with great promise comes great responsibility (sorry to mix superhero metaphors), and researchers like Abbosh understand the need to deliver tests with as high of accuracy as possible to merit clinical use. Abbosh’s test is not there yet, but he says it is still “early days” in his research.
Currently, only a handful of urine-based cancer detection tests are approved by the U.S. Food and Drug Administration (FDA), including tests for bladder and prostate cancers. These tests need to show that they can accurately measure both sensitivity—the percentage of people correctly diagnosed with disease—as well as specificity—the percentage of people without disease correctly identified.
Research into expanding the options for urine-based cancer detection, however, is not in short supply. A review in the International Journal of Cancer found 924 journal articles published between January 2010 and January 2022 that mentioned the use of urine biomarkers to detect various forms of cancer, including thyroid, lung, liver, biliary tract, kidney, prostate, head and neck, breast, gastric, pancreatic, colorectal, endometrial, ovarian, cervical, and urothelial.
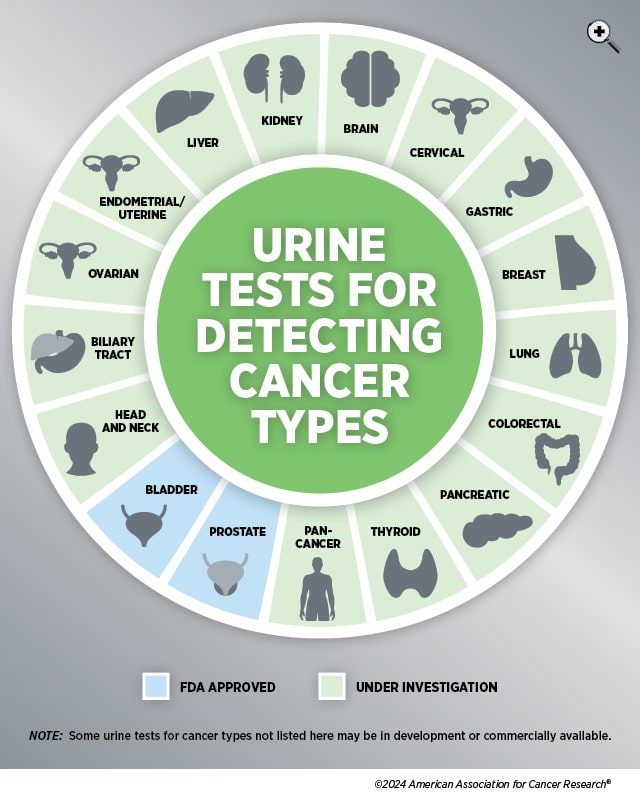
More recently, the Advanced Research Projects Agency for Health (ARPA-H), an independent agency within the U.S. Department of Health and Human Services to support biomedical and health breakthroughs, announced its goal to develop an at-home self-screening test that can detect 30+ cancers in stage 1 using urine or breath samples. This program, called the Platform Optimizing SynBio for Early Intervention and Detection in Oncology (POSEIDON), is part of the Biden Cancer Moonshot’s goal “to end cancer as we know it.”
Other recent studies have examined researchers’ work into using urine tests to detect or monitor the progress of cancer in the lungs, ovaries, pancreas, bladder, prostate, and more.
Lung Cancer: Don’t Forget the Nevers, the Nons, and the Nos
About 10% to 20% of lung cancer cases in the United States are in never-smokers, but the U.S. Preventive Services Task Force (USPSTF) does not currently recommend screening using low-dose computed tomography (LDCT) for those who have never smoked. Researchers interested in developing a screening option that includes non- and never-smokers wanted to see if they could identify biomarkers for lung cancer in urine specific to these individuals.
In a study published in Clinical Cancer Research, a journal of the AACR, the researchers looked at two biomarkers they previously connected to lung cancer—creatine riboside (CR) and N-acetylneuraminic acid (NANA)—and compared urine samples of nonsmokers and active smokers, including some with non-small cell lung cancer (NSCLC) and others in a control group. Levels of both CR and NANA were significantly elevated in both nonsmokers and smokers with lung cancer compared to the control groups. Additionally, the median levels of CR and NANA were elevated in nonsmoker cases with lung cancer who did not report exposure to secondhand smoke or childhood parental smoking, which the authors said could indicate these biomarkers are not tied to tobacco smoking.
The authors suggest this method could either be used as a screening option on its own or to help identify which individuals should undergo LDCT, even if they don’t meet current USPSTF guidelines.
But given that not everyone may have access to LDCT, another set of researchers wanted to design a lung cancer screening method that could potentially address this disparity. In a study published in Science Advances, researchers tested the use of what they coined PATROL (point-of-care aerosolizable nanosensors with tumor-responsive oligonucleotide barcodes) in mouse models with lung cancer.
First, an inhaler or nebulizer is used to deliver activity-based nanosensors (ABNs) engineered with DNA barcodes into the lungs. These DNA barcodes separate from the ABNs and enter the mouse’s circulation if they encounter enzymes associated with early lung adenocarcinoma. Eventually, the barcodes are concentrated in the urine via the kidneys, so when the mouse pees on the specially designed paper-based test strip, it can determine if the barcodes that made it out of the body indicate signs of lung cancer. In mice, results could be achieved within 20 minutes, but the test still needs to be validated in humans.
Ovarian Cancer: Gold Fingerprint
Whereas a screening option is available for lung cancer, no such options are currently available for ovarian cancer. But considering the five-year survival rate for ovarian cancer is 50.9%, researchers have been exploring ways to catch the disease earlier, including blood and urine tests.
In a study published in ACS Sensors, researchers examined whether certain small molecules, called peptides, that are known to exist in the urine of ovarian cancer patients could be more easily detected than via the often complicated and costly methods currently used for peptide detection. The researchers tried a variation of nanopore sensing, a method used for DNA or RNA sequencing in which molecules are passed through a tiny pore to measure the changes in electronic current. By blocking the pore with gold nanoparticles, the researchers hypothesized certain peptides would stick to the gold particle and produce a unique current signature that they compared to a fingerprint.
Using research peptides, they found this “fingerprinting” approach could detect certain peptides associated with ovarian cancer in the LRG-1 protein such as P19C2. To test this approach in urine, they spiked a sample from an ovarian cancer patient with P19C2. There was “considerable overlap” between the signatures they previously observed for P19C2 and what they saw in the urine sample. While this approach requires further study, the researchers hope it could eventually be paired with family history, transvaginal ultrasound, and/or blood tests to help with earlier detection.
Pancreatic Cancer: Early Results for Earlier Detection
The five-year survival rate for pancreatic cancer is even lower at 12.8%, in part due to a lack of screening options available to catch the disease early. At the AACR Special Conference in Cancer Research: Advances in Pancreatic Cancer Research, held September 15-18, a team of researchers presented the latest updates on their work to develop a potential urine test for this cancer type.

Previously, the team showed that a urinary biomarker panel, comprised of the proteins LYVE1, REG1B, and TFF1, was able to detect pancreatic cancer in stages 1 through 2 with a sensitivity and specificity above 85% in samples collected from previously diagnosed patients. Later, they used prediagnostic samples to test the predictive performance of this urinary panel both alone and in combination with CA19-9 in blood samples—a known biomarker for pancreatic cancer—and found the urinary panel was able to detect pancreatic cancer up to two years prior to diagnosis.
Now, they are recruiting for the UroPanc Trial to evaluate the test in two cohorts: one containing asymptomatic individuals with a family history or genetic syndromes related to pancreatic cancer and the other containing patients with symptoms associated with pancreatic cancer. While recruiting is ongoing, at the conference they presented an interim analysis from 825 patients who have already had their urine samples assayed. So far, the test has shown a sensitivity of 91%, a specificity of 86%, and an accuracy of cancer detection of 90%.
Tatjana Crnogorac-Jurcevic, MD, PhD, of Barts Cancer Institute at Queen Mary University of London, said they have also found promising urinary biomarkers for cholangiocarcinoma that they are working to further validate and would like to find a signature for gastric cancer, as well.
Colorectal Cancer: Screening, Treatment, or Both?
Between fecal immunochemical tests (FIT), a newly approved blood test, and colonoscopies, detection methods for colorectal cancer are not lacking, but what about a screening option that could also serve as a delivery method for treatment?
Researchers engineered bacteria—E. coli Nissle 1917 (EcN)—to seek out polyps in mouse models and had success in both detecting their presence and reducing their size, but in separate instances, according to results published in Nature Communications. While most polyps are benign, some can be precancerous and grow into cancer if they aren’t removed. The researchers designed EcN to produce salicylate if polyps were present. When they tested this version of EcN in mouse models with polyps, the salicylate levels in the urine were five times higher than baseline measurements. Meanwhile, healthy mouse models saw no difference in salicylate levels over time.
The researchers next wanted to see if EcN could be engineered to treat polyps. They designed a version of the bacteria that could maximize the delivery of multiple payloads of immunotherapy and found that it helped reduce the size of polyps by around 47% throughout the small intestine. The researchers hope these findings can lay the groundwork for preclinical and clinical testing of EcN to detect and treat colorectal cancer.
Prostate Cancer: Aiming for Higher Grade Accuracy
Prostate-specific antigen (PSA)-based blood tests—one of the current methods used to screen for prostate cancer—have been found to reduce the mortality of the disease, but they can also lead to invasive biopsies in men without cancer. Multiparametric magnetic resonance imaging (mpMRI) can be one option, if available, to detect tumors in men with elevated PSA levels and avoid invasive biopsy, but interpretation of imaging can be subjective and lead to diagnosis of prostate cancer being missed in some patients or misdiagnosed in others.
Researchers explored whether they could improve upon a urine-based test they previously developed, MyProstateScore, to better identify more aggressive forms of prostate cancer and limit the need for invasive biopsies following PSA testing. Prostate cancer is given a grade between 1 and 5, with a higher number indicating a greater likelihood the cancer will grow and spread. Since the development of MyProstateScore, new biomarkers expressed in higher grade prostate cancer have been identified. By incorporating these learnings into MyProstateScore 2.0, the gene panel used for indicating signs of disease expanded from two to 18.
When evaluated in two cohorts of men, each with over 700 participants, MyProstateScore 2.0 provided 99% sensitivity and 99% negative predictive value (NPV)—when a negative result corresponds with no actual disease—for prostate cancer grade 3 and NPVs between 95% and 99% for grade 2 or higher, according to results published in JAMA Oncology. The researchers estimated that the use of this test could have helped 35% to 51% of the study’s participants safely avoid additional unnecessary imaging or biopsy.
MyProstateScore 2.0 is currently commercially available, but not FDA-approved.
Bladder Cancer: Urine and Blood Stronger Together
During a session at the AACR Special Conference on bladder cancer, Lars Dyrskjøt, PhD, of the Aarhus University Hospital in Denmark, made the case that blood and urine tests can make a powerful combo. In a study previously published in Clinical Cancer Research, his team used liquid biopsy to test for small pieces of DNA that are released when tumors die. When detected in the blood, this is known as circulating tumor DNA (ctDNA). Meanwhile, traces of tumor in the urine are known as utDNA.
“ctDNA has a half-life of about two hours, so that makes it possible to make some real-time DNA monitoring of the tumor burden during treatment response,” Dyrskjøt explained.
Dyrskjøt and his team measured ctDNA and utDNA in patients with muscle-invasive bladder cancer both prior to and during neoadjuvant chemotherapy. Levels of ctDNA both predicted and indicated response to treatment as well as corresponded to regression-free survival when measured both before and after treatment. Levels of utDNA were most effective in predicting treatment response and regression-free survival. However, Dyrskjøt noted that the findings were strongest when data from blood and urine were pulled together. A negative sample of both pointed toward an “exceptional good outcome” for patients, he said.
Dyrskjøt added that these results could help determine when to de-escalate treatment or whether to consider additional treatment options such as immunotherapy.
Head and Neck Cancer: The Long and Short of It
Another set of researchers wanted to look at whether cell-free DNA that is filtered from the bloodstream to the kidneys and into urine, which they called transrenal cell-free DNA (TR-ctDNA), could provide information from a variety of cancers throughout the body, according to a paper published in JCI Insight. Previous efforts into developing a urine-based test to extract this information have had mixed results. These researchers wanted to test if size was the culprit.
Hypothesizing that the length of TR-ctDNA fragments in urine were much shorter than most tests were designed to capture, the researchers developed a droplet digital PCR (ddPCR)-based assay to detect ultrashort TR-ctDNA. They focused on identifying HPV16, a subtype shared by 90% of patients with HPV+ oropharyngeal squamous cell carcinoma (OPSCC). Urine and blood samples were collected from 32 HPV+ OPSCC patients prior to treatment, and the results in detecting HPV16 in the blood and urine samples were comparable. Further, HPV16 was not detected in any of the 12 control samples without HPV+ OPSCC.
In a pilot study, the researchers used the assay in four HPV+ OPSCC patients who received treatment, three with chemoradiation and one with surgical resection. In three of the four cases, the ddPCR assay detected cancer recurrence earlier than clinical diagnosis.
The researchers hope this proof-of-concept study encourages others to take a long-term view of the potential that ultrashort TR-ctDNA—and urine in general—holds as a way to expand access to noninvasive cancer detection and monitoring.



