Cancer Discovery Explores New Horizons for Cancer Research in China
A special collection in the November 2024 issue of Cancer Discovery, “New Horizons for Cancer Research: Innovative Ideas by Leading Chinese Researchers,” was motivated by a simple philosophy—that good ideas don’t have geographic boundaries.
“Cancer researchers in China, who now represent the largest cancer research workforce in the world, are making incredible strides against cancer,” said Robert Kruger, PhD, senior executive editor of Cancer Discovery, a journal of the American Association for Cancer Research (AACR), and editorial director for the AACR journals. “Given Cancer Discovery’s longstanding reputation of showcasing major advances in cancer research, we felt it important for the journal to magnify the pioneering ideas that Chinese researchers are exploring to our readers across the globe.”
Earlier this year, the journal issued a call for commentaries on BioArt, a social media account popular with Chinese researchers. As part of the initiative, Cancer Discovery’s executive editor, Elizabeth McKenna, PhD, participated in a live social media discussion to answer researchers’ questions about the journal and the types of commentaries it was seeking.
“We were particularly interested in commentaries exploring new and thought-provoking ideas on emerging topics,” said McKenna. “We are excited to amplify the voices of Chinese thought leaders to new audiences and promote the exchange of ideas through the pages of Cancer Discovery. Too often, Western and Eastern research communities are siloed, so we hope this collection will help bridge these communities and foster new conversations and research approaches.”
In the end, nine commentaries were selected, covering topics such as using machine learning to improve cancer risk prediction, integrating traditional Chinese medicine with Western medicine, developing new ways to target p53, and exploring the non-H. pylori microbiome in gastric cancer, among others. Keep reading to learn about some of the commentaries, and be sure to check out the full collection here.
A Call for Innovative Translational and Clinical Research to Address China’s Unique Cancer Landscape

Government initiatives have accelerated cancer research in China in recent years, but more focus is needed on research that addresses China’s particular cancer landscape, wrote Nan Sun, PhD; Jie He, MD, PhD; and colleagues in the first commentary of the collection.
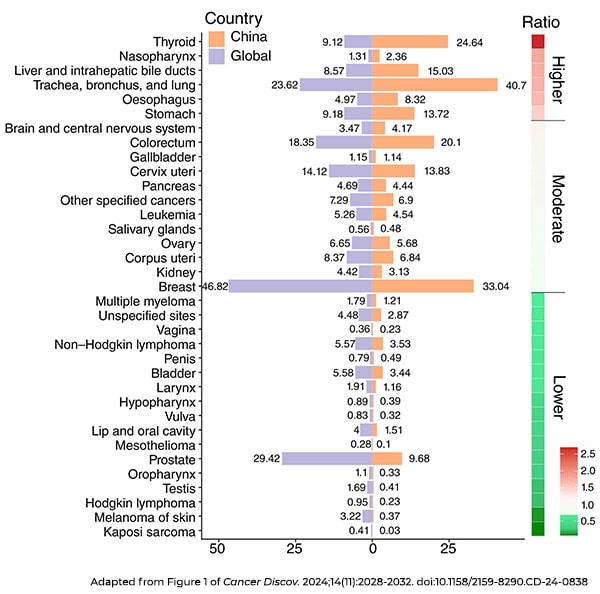
The authors argued that China has features of both developed and developing countries, and this distinction has led to a unique set of cancers in the country, including higher rates of thyroid cancer, nasopharynx cancer, liver and intrahepatic bile duct cancers, lung cancer, esophageal cancer, and gastric cancer, compared with the global average.

Clinical research in China, however, has largely focused on other cancer types. “In other words, there remains a significant disparity between the clinical demands in China and the recent remarkable advancements in cancer research within the country,” the authors explained.
To mitigate this discrepancy, Sun, He, and colleagues called for increased financial incentives from the government to promote cooperation between the pharmaceutical industry and basic researchers, as well as stronger intellectual property laws to encourage the development of first-in-class therapeutics. With this support, researchers should work toward developing and testing drugs specific to the cancer types prevalent in China, the authors continued, utilizing tools such as artificial intelligence to expedite drug discovery and databases to facilitate data sharing and collaboration.
Advancing Cancer Prevention Through an AI-based Integration of Traditional and Western Medicine
The multifaceted nature of cancer development complicates prevention and early detection efforts, said Shao Li, MD, PhD, and colleagues in another commentary. They posited that a deeper understanding of the various levels of tumor development—molecular to organismal—and how they interact and evolve with time is required to improve cancer prevention and detection, particularly in China, where fewer than 30% of breast, colorectal, and lung cancers are detected early.

To this end, the authors proposed using artificial intelligence (AI) to integrate traditional Chinese medicine (TCM) with modern Western medicine. The holistic nature of TCM, which is rooted in understanding whole body changes, “aligns with the current shift in Western medicine from reductionism to systematism, offering new perspectives for cancer prevention research and providing a valuable complement to Western medical practices,” they noted.
Li and colleagues suggested that AI could analyze large multimodal data sets collected from patients who received either TCM or Western medicine to identify bodily changes and molecular markers associated with cancer and then integrate these to formulate accurate risk prediction models. Further, analyzing the effects of various herbal remedies and Western medicines through self-reported use of traditional medicine, electronic medical records, and other sources could identify potential strategies for cancer interception.
“The development of AI technology empowers us to deepen our understanding of cancer development and translate related data and insights from traditional medicine into actionable strategies in cancer prevention,” the authors summarized.
Early Cancer Detection Through Comprehensive Mapping of Dynamic Tumorigenesis
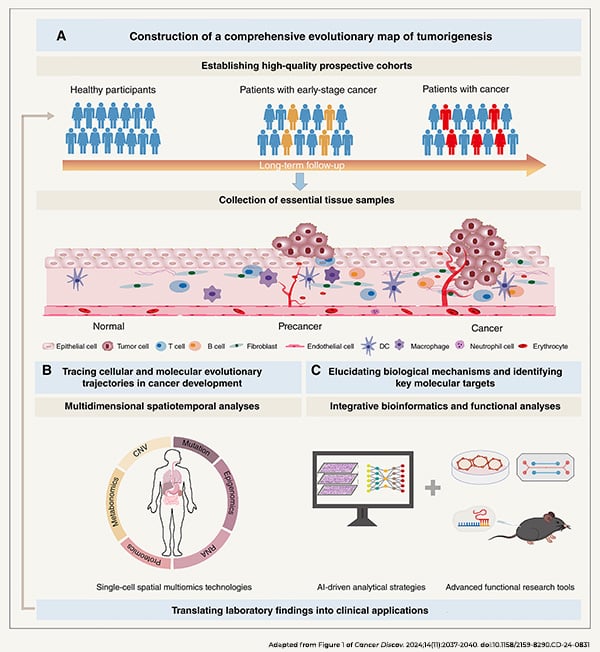
The third commentary of the series continued the theme of improving cancer risk prediction, with Chen Wu, MD, PhD, and colleagues noting the need for spatial mapping of cell populations and their interactions as normal tissue evolves to precancer and then to cancer.

“Such advancements will enable the identification of potential molecular targets for early detection, thereby enhancing our ability to build prediction models for identifying high-risk individuals,” the authors wrote.
They explained that a cancer evolution map will require a prospective cohort of healthy individuals who are followed over time with detailed documentation of demographics, lifestyle, medical history, and psychological factors. They suggested that researchers utilize spatiotemporal multiomics technologies to study multifaceted changes to the tissue during cancer development and that they periodically collect blood and urine samples to track genetic features, metabolic markers, and environmental exposures. Additionally, the authors recommended that the research be conducted by an interdisciplinary team of epidemiologists, clinicians, cancer biologists, and data scientists.
The analysis and validation of these large data sets will benefit from machine learning approaches and cutting-edge model systems such as organ-on-a-chip and advanced organoids, Wu and colleagues noted.
Building an Organ-wide Macroscopic View of Cancer Hallmarks

While a tumor may form in a particular tissue, it exists within the context of the whole body. Suling Liu, PhD; Zhihua Liu, PhD; Shengtao Zhou, MD; and their colleagues argued that crosstalk between different organs could influence cancer progression, presenting opportunities for therapeutic intervention.
Crosstalk between organs may occur through extracellular vesicles, metabolites, hormones, or hematopoietic cells, which can be released from one organ to another to deliver signals that promote tumorigenic processes at the receiving site. These signals may even influence response to treatment and cancer-related complications, including cachexia, the authors explained.

One example of such influence is the impact of gut bacteria on cancers outside the gastrointestinal tract due, in part, to the release of metabolites. The release of the metabolite indole-3-acetic acid by gut bacteria, for instance, is associated with greater likelihood of chemotherapy response in patients with pancreatic cancer.

While any of the mediators of organ crosstalk are potentially actionable, the authors suggested that “microbiota and related metabolites are optimal to be targeted as they are easy to identify and to be functionally characterized, and most importantly, could be readily modulated.” However, they noted that additional preclinical and clinical studies are needed to test the efficacy and safety of targeting organ crosstalk mediators for cancer treatment.