Personalized Vaccines and Beyond: Confronting the Complex Biology of Kidney Cancer
Breakthroughs in kidney cancer research, including new insights into the tumor-immune microenvironment and the identification of metabolic vulnerabilities, are reshaping the future of treatment, creating opportunities for therapies tailored specifically to individual patients. These cutting-edge developments in personalized vaccines targeting tumor-specific mutations, novel cell therapies, and biomarkers that can predict treatment responses, could help to revolutionize kidney cancer care and significantly enhance patient outcomes and quality of life.
Personalized Neoantigen Vaccines: A Potential Leap Forward for Kidney Cancer
At the forefront is a remarkable personalized neoantigen vaccine approach that targets mutated protein fragments known as neoantigens that are expressed exclusively by cancer cells. This strategy has been effective for immunogenic tumors like melanoma and lung cancers that often carry many mutations, but until now, had yet to show much promise in cancers that typically harbor fewer mutations, including kidney cancer.

In a recent phase I trial, nine patients with advanced clear cell renal cell carcinoma (ccRCC) received peptide-based vaccines tailored to their tumors’ specific mutations after their tumors were fully resected. Renal cell carcinoma is the most common form of kidney cancer, accounting for approximately 90% of kidney cancer cases, and the clear cell subtype accounts for roughly 80% of RCC cases.
Of the nine patients in the trial, five of whom were also treated with the immune checkpoint inhibitor (ICI) ipilimumab (Yervoy), none experienced disease relapse at a median follow-up of 40.2 months. Eight of the nine patients have had at least two years of follow-up, and the ninth unfortunately died from an unrelated cause, after no evidence of disease was observed at their final, 19-month follow-up. Impressively, five of these patients have gone more than three years without recurrence. Additionally, the vaccines successfully induced durable T-cell responses, predominantly from memory CD4-positive cells that had already encountered antigen.
One aspect of this promising approach involved targeting driver mutations, genetic alterations that play a central role in enabling tumor growth and survival. A prime example in kidney cancer is the tumor suppressor gene VHL. Mutations that inactivate VHL and prevent it from fulfilling its tumor suppressor duties are a hallmark of ccRCC, through their impact on pathways crucial for tumor development. But mutant VHL-derived peptides can also provide targets for the immune system, as demonstrated by a pioneering 2010 pilot study in which a peptide vaccine elicited immune responses against mutant VHL in four of the five patients treated. Building upon this foundation, the recent personalized neoantigen vaccine strategy incorporated mutate VHL in addition to several other mutant driver targets that the patients’ kidney cancers harbored.
Cell Therapy and the Solid Tumor Microenvironment
Besides personalized vaccines, cell therapies have gained some traction in treating kidney cancer. One example is an innovative CAR T-cell therapy targeting the CD70 receptor. Unlike currently approved CAR T-cell therapies, which are created from patients’ own harvested immune cells, this CD70 CAR T-cell therapy is created using healthy donor T cells, offering a more readily available and predefined cell therapy product. Additionally, in contrast to neoantigens that are unique to individual tumors, CD70 is a tumor-associated antigen linked to immunosuppression and frequently overexpressed in ccRCC.
In a phase I study, the CD70-targeting therapy, CTX130, prevented disease progression in 13 of 16 patients with advanced ccRCC. Most patients experienced disease stabilization, and one patient experienced complete elimination of their tumor and remained disease-free three years later, underscoring the potential of engineered cell therapies.
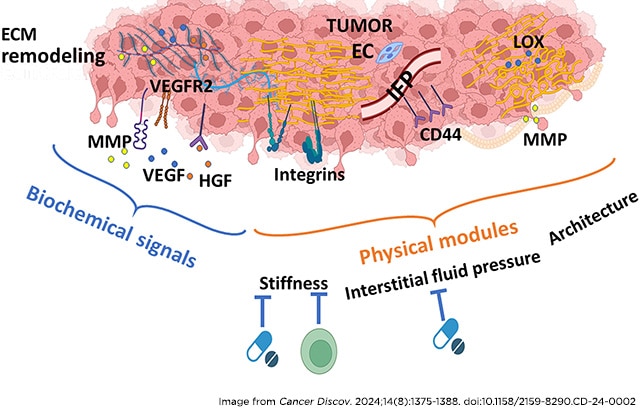
The development of superior therapies will require navigating the complex and uniquely challenging tumor microenvironment of kidney cancers. Recent research underscores kidney tumors’ extensive extracellular matrix remodeling and unusually high interstitial fluid pressure, which can create a physical barrier that limits the distribution of drug delivery within tumors and promotes resistance. Understanding and targeting these aspects of tumors could significantly improve the effectiveness of current and future therapies.
Metabolic Vulnerabilities as Therapeutic Targets
Metabolism has also emerged as a critical factor in kidney cancer biology, revealing numerous potential therapeutic targets. For instance, researchers discovered that ccRCC cells exploit a mitochondrial enzyme, MTHFD2, to enhance glycosylation of the growth-promoting protein c-MYC and thereby drive resistance to the commonly used targeted therapy sunitinib (Sutent). Blocking MTHFD2, therefore, could restore drug sensitivity.
Another study showed that ccRCC tumors accumulate copper, which fuels mitochondrial energy production and tumor growth. Interrupting copper metabolism could weaken tumor cells, opening another avenue for therapeutic intervention.
Lipid metabolism is another critical axis of ccRCC survival. ccRCC tumors depend on elevated lipid uptake and storage, which provide essential energy reserves and structural components that help tumor cells endure cellular stress. In particular, metastatic ccRCC cells manipulate lipid-related receptors such as GPR1 and CMKLR1 to enhance lipid accumulation, bolstering their ability to resist stress-induced death.
Within these lipid stores, cholesterol is the most abundant component, playing a central role in maintaining cellular homeostasis. To manage this dependency, ccRCC tumors rely on enzymes like HSD3B7, which regulate cholesterol and bile acid metabolism to prevent the buildup of toxic cholesterol metabolites. Targeting this lipid network, both at the receptor level and within downstream metabolic pathways, represents a promising therapeutic strategy that could disrupt tumor survival mechanisms and trigger cancer cell death.
Likewise, modulating tryptophan metabolism by inhibiting the enzyme PABPC1L could enhance immune responses by preventing immune suppression, further boosting the effectiveness of immunotherapies.
Biomarkers: Paving the Path to Precision Medicine
As treatments become more specialized and complex, biomarkers will be crucial for pairing patients with the right treatments and predicting therapy responses. To that end, scientists identified immune cell signatures, such as certain subpopulations of natural killer cells and regulatory T cells in the blood of metastatic kidney cancer patients, that could serve as predictors of response to immune checkpoint blockade. Meanwhile, CA-125 has emerged as a promising noninvasive biomarker in renal medullary carcinoma, a rare and aggressive kidney cancer linked to sickle cell trait, correlating with metastatic burden and disease progression
Collectively, these developments—from personalized neoantigen vaccines and CAR T therapies to deepened understanding of the tumor-immune microenvironment and metabolic vulnerabilities—signal a convergence towards precision-driven kidney cancer care. The insights gained position researchers and clinicians to pursue treatments offering genuine leaps forward in survival and quality of life for kidney cancer patients.