Editors’ Picks, March 2025: Cancer Cell Senescence, Ultraviolet Protection, and More
As we close out the first quarter of 2025, we are excited to present this month’s Editors’ Picks. March’s selections from the editors of the 10 American Association for Cancer Research (AACR) journals highlight studies on the immunostimulatory impact of extracellular vesicles secreted by senescent cancer cells, a molecule that might protect cells from ultraviolet radiation’s harmful effects, strategies for manipulating mesenchymal-like cancer cells, and more. As always, these studies will be freely available for a limited time.
Journal: Blood Cancer Discovery
The Crossroads of Clonal Evolution, Differentiation Hierarchy, and Ontogeny in Leukemia Development
Transformative technologies to sequence tumor genomes at large scale and single-cell resolution have exposed the repertoire of genetic alterations that are present in leukemia genomes, the timing of their acquisition, and patterns of their co-occurrence. In parallel, single-cell multiomics technologies are allowing us to map the differentiation paths and hierarchical structures of malignant cells and giving us a glimpse into hematopoietic development in prenatal life. We propose that interrogating how the genetic evolution, differentiation hierarchy, and ontogeny of malignant myeloid cells intersect with each other, using new experimental systems and multimodal technologies, will fuel the next generation of research breakthroughs.
Significance: In recent years, remarkable technological advances have illuminated aspects of the pathogenesis of myeloid malignancies—yet outcomes for patients with these devastating diseases have not significantly improved. We posit that a synthesized view of the three dimensions through which hematopoietic cells transit during their healthy and diseased life—clonal evolution, stem cell hierarchy, and ontogeny—promises high yields in new insights into disease pathogenesis and new therapeutic avenues.
This article was featured on the cover of the March issue.
Journal: Cancer Discovery
PKN2 Is a Dependency of the Mesenchymal-like Cancer Cell State
Cancer cells exploit a mesenchymal-like transcriptional state (MLS) to survive drug treatments. Although the MLS is well characterized, few therapeutic vulnerabilities targeting this program have been identified. In this study, we systematically identify the dependency network of mesenchymal-like cancers through an analysis of gene essentiality scores in ∼800 cancer cell lines, nominating a poorly studied kinase, PKN2, as a top therapeutic target of the MLS. Coessentiality relationships, biochemical experiments, and genomic analyses of patient tumors revealed that PKN2 promotes mesenchymal-like cancer growth through a PKN2−SAV1−TAZ signaling mechanism. Notably, pairing genetic PKN2 inhibition with clinically relevant targeted therapies against EGFR, KRAS, and BRAF suppresses drug resistance by depleting mesenchymal-like drug-tolerant persister cells. These findings provide evidence that PKN2 is a core regulator of the Hippo tumor suppressor pathway and highlight the potential of PKN2 inhibition as a generalizable therapeutic strategy to overcome drug resistance driven by the MLS across cancer contexts.
Significance: This work identifies PKN2 as a core member of the Hippo signaling pathway, and its inhibition blocks YAP/TAZ-driven tumorigenesis. Furthermore, this study discovers PKN2−TAZ as arguably the most selective dependency of mesenchymal-like cancers and supports specific inhibition of PKN2 as a provocative strategy to overcome drug resistance in diverse cancer contexts.
This article was highlighted and featured on the cover of the March issue, in addition to a commentary related to this study.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Estradiol and estrone are well-established risk factors for postmenopausal breast cancer. Experimental evidence suggests that specific estrogen metabolites, produced via irreversible hydroxylation of estrone and estradiol at position 2 or 16, may independently influence carcinogenesis.
Methods: We performed a nested case–control study of breast cancer (328 breast cancer cases; 639 controls) among postmenopausal women within the Nurses’ Health Study to examine the role of estrogens and estrogen metabolites (jointly referred to as EM). Plasma concentrations of each EM (unconjugated + conjugated forms) were measured by LC-MS/MS. Multivariable conditional logistic regression, adjusting for breast cancer risk factors, estimated relative risks (RR) and 95% confidence intervals of breast cancer across quintiles of individual EM, EM pathways, and pathway ratios. Associations by estrogen receptor (ER) and progesterone receptor (PR) status were analyzed by unconditional logistic regression.
Results: Estradiol and estrone were strongly associated with increased breast cancer risk [estradiol: RRQ5 vs. Q1 (95% confidence interval) = 2.64 (1.64–4.26); estrone: 2.78 (1.74–4.45); both P-trends <0.001]. The 2-hydroxylation pathway was strongly associated with risk [RRQ5 vs. Q1 = 3.09 (1.81–5.27); P-trend <0.001] and remained so after adjusting for unconjugated estradiol [RRQ5 vs. Q1 = 2.23 (1.25–3.96); P-trend = 0.01]. Although the 16-hydroxylation pathway was modestly associated with risk [RRQ5 vs. Q1 = 1.62 (1.03–2.54); P-trend = 0.01], the association was attenuated after unconjugated estradiol adjustment [RRQ5 vs. Q1 = 1.24 (0.77–1.99); P-trend = 0.19]. Similar positive associations with the 2-pathway and 16-pathway were observed for ER+/PR+ and ER−/PR− tumors.
Conclusions: In this cohort of postmenopausal women, 2-hydroxylation of estrone and estradiol was associated with increased breast cancer risk, independent of unconjugated estradiol.
Impact: These results highlight the need to revisit the role of estrogen metabolism in breast cancer etiology and prevention.
This article was highlighted in the March issue, in addition to a commentary related to this study.
Journal: Cancer Immunology Research
Despite advances in cancer immunotherapy, such as targeting the PD-1/PD-L1 axis, a substantial number of patients harbor tumors that are resistant or relapse. Selective engagement of T-cell co-stimulatory molecules with bispecific antibodies may offer novel therapeutic options by enhancing signal 1–driven activation occurring via T-cell receptor engagement. In this study, we report the development and preclinical characterization of NI-3201, a PD-L1×CD28 bispecific antibody generated on the κλ-body platform that was designed to promote T-cell activity and antitumor function through a dual mechanism of action. We confirmed that NI-3201 blocks the PD-L1/PD-1 immune checkpoint pathway and conditionally provides T-cell co-stimulation via CD28 (signal 2) when engaging PD-L1+ tumors or immune cells. In systems with signal 1–primed T cells, NI-3201 enhanced potent effector functionality: in vitro through antigen-specific recall assays with cytomegalovirus-specific T cells and in vivo by inducing tumor regression and immunologic memory in tumor-associated antigen–expressing MC38 syngeneic mouse models. When T-cell engagers were used to provide synthetic signal 1, the combination with NI-3201 resulted in synergistic T cell–dependent cytotoxicity and potent antitumor activity in two humanized mouse tumor models. Nonhuman primate safety assessments showed favorable tolerability and pharmacokinetics at pharmacologically active doses. Quantitative systems pharmacology modeling predicted that NI-3201 exposure results in antitumor activity in patients, but this remains to be investigated. Overall, this study suggests that by combining PD-L1 blockade with safe and effective CD28 co-stimulation, NI-3201 has the potential to improve cancer immunotherapy outcomes, and the clinical development of NI-3201 for PD-L1+ solid tumors is planned.
Journal: Cancer Prevention Research
This study aimed to assess how ursolic acid (UA) can protect human skin keratinocytes from damage caused by UVB radiation. Utilizing an omics-based approach, we characterized the features of photodamage and investigated the potential of UA to reverse HaCaT cell subpopulation injury caused by UVB radiation. The most significant changes in metabolite levels after UA treatment were in pathways associated with phosphatidylcholine biosynthesis and arginine and proline metabolism. Treatment with UA can reverse the levels of certain metabolites, including creatinine, creatine phosphate, and succinic acid. Pathways activated by UA treatment in UVB-irradiated HaCaT cells were associated with several biological processes, including the positive regulation of protein modification process, cell division, and enzyme-linked receptor protein signaling pathway. Treatment with UA demonstrates the capability to mitigate the effects of UVB radiation on specific genes, including S100 calcium-binding protein A9 and IL6 receptor. DNA/CpG methylation indicates that UA can partially reverse some of the alterations in the UVB-induced CpG methylome. Utilizing integrated RNA sequencing and methylation sequencing data, starburst plots illustrate the correlation between mRNA expression and CpG methylation status. UA potentially influences the metabolic pathway of glycerophospholipid metabolism by modulating the expression of several key enzymes, including phospholipase A2 group IIA and lipin 2. Altogether, these results indicate that UVB radiation induces metabolic reprogramming, epigenetic changes, and transcriptomic shifts. Meanwhile, UA demonstrates the capacity to inhibit or reduce the severity of these alterations, which may underlie its potential protective role against skin damage caused by UVB exposure.
Prevention Relevance: Our research indicates that UA has the potential to mitigate or lessen the impact of UVB radiation, which is known to cause metabolic reprogramming, epigenetic alterations, and transcriptomic changes. These effects could be responsible for UA’s possible protective function against skin damage induced by UVB exposure.
This story was featured on the cover of the March issue.
Journal: Cancer Research (March 1 issue)
Senescence is a nonproliferative survival state that cancer cells can enter to escape therapy. In addition to soluble factors, senescence cells secrete extracellular vesicles (EV), which are important mediators of intercellular communication. To explore the role of senescent cell (SC)–derived EVs (senEV) in inflammatory responses to senescence, we developed an engraftment-based senescence model in wild-type mice and genetically blocked senEV release in vivo, without significantly affecting soluble mediators. SenEVs were both necessary and sufficient to trigger immune-mediated clearance of SCs, thereby suppressing tumor growth. In the absence of senEVs, the recruitment of MHC-II+ antigen-presenting cells (APC) to the senescence microenvironment was markedly impaired. Blocking senEV release redirected the primary target of SC signaling from APCs to neutrophils. Comprehensive transcriptional and proteomic analyses identified six ligands specific to senEVs, highlighting their role in promoting APC–T cell adhesion and synapse formation. APCs activated CCR2+CD4+ TH17 cells, which seemed to inhibit B-cell activation, and CD4+ T cells were essential for preventing tumor recurrence. These findings suggest that senEVs complement the activity of secreted inflammatory mediators by recruiting and activating distinct immune cell subsets, thereby enhancing the efficient clearance of SCs. These conclusions may have implications not only for tumor recurrence but also for understanding senescence during de novo carcinogenesis. Consequently, this work could inform the development of early detection strategies for cancer based on the biology of cellular senescence.
Significance: Chemotherapy-treated senescent tumor cells release extracellular vesicles that trigger an immune response and suppress tumor recurrence.
A commentary related to the study was published in the March 1 issue.
Journal: Cancer Research (March 15 issue)
Radiotherapy is an integral component in the treatment of many types of cancer, with approximately half of patients with cancer receiving radiotherapy. Systemic therapy applies pressure that can select for resistant tumor subpopulations, underscoring the importance of understanding how radiation impacts tumor evolution to improve treatment outcomes. We integrated temporal genomic profiling of 120 spatially distinct tumor regions from 20 patients with undifferentiated pleomorphic sarcomas (UPS), longitudinal circulating tumor DNA analysis, and evolutionary biology computational pipelines to study UPS evolution during tumorigenesis and in response to radiotherapy. Most unirradiated UPSs displayed initial linear evolution, followed by subsequent branching evolution with distinct mutational processes during early and late development. Metrics of genetic divergence between regions provided evidence of strong selection pressures during UPS development that further increased during radiotherapy. Subclone abundance changed after radiotherapy with subclone contraction tied to alterations in calcium signaling, and inhibiting calcium transporters radiosensitized sarcoma cells. Finally, circulating tumor DNA analysis accurately measured subclone abundance and enabled noninvasive monitoring of subclonal changes. These results demonstrate that radiation exerts selective pressures on UPSs and suggest that targeting radioresistant subclonal populations could improve outcomes after radiotherapy.
Significance: Radiotherapy mediates tumor evolution by leading to the expansion of resistant subclonal cancer cell populations, indicating that developing approaches to target resistant subclones will be crucial to improve radiotherapy response.
This article was featured on the cover of the March 15 issue.
Journal: Clinical Cancer Research (March 1 issue)
Purpose: Combinations of immune checkpoint inhibitors and nab-paclitaxel have improved outcomes in advanced urothelial carcinoma and muscle-invasive bladder cancer. This study evaluates the safety and efficacy of tislelizumab combined with low-dose nab-paclitaxel in extensive very high–risk non–muscle-invasive bladder cancer.
Patients and Methods: TRUCE-02 was a single-arm phase II trial that included 63 patients with visually incomplete resection and/or high-volume high-grade T1 tumors (with or without carcinoma in situ) who were ineligible for or declined radical cystectomy. Patients received intravenous tislelizumab (200 mg on day 1) and nab-paclitaxel (200 mg on day 2) every 3 weeks, with assessment approximately 3 months after initial administration. The primary endpoint was the complete response (CR) rate of high-risk disease. Main secondary endpoints included safety and duration of CR.
Results: The safety analysis included all 63 patients, and the efficacy analysis included 59 patients. Thirty-seven patients (62.7%; 95% confidence interval, 49.1%–75.0%) achieved a CR of high-risk disease, with a 24-month sustained response rate of 96.3% (95% confidence interval, 89.4%–100.0%). Grade 3 to 4 treatment-related adverse events occurred in nine patients (14%), with no fatal events reported.
Conclusions: Tislelizumab plus low-dose nab-paclitaxel was well tolerated and showed promising antitumor activity, making it a potential alternative for patients with extensive very high–risk non–muscle-invasive bladder cancer who are ineligible for or decline radical cystectomy.
Journal: Clinical Cancer Research (March 15 issue)
Purpose: Signal transducer and activator of transcription 3 is a transcription factor that is essential for the survival and immune sequestration of cancer cells. We conducted a phase I study of TTI-101, a first-in-class, selective small-molecule inhibitor of signal transducer and activator of transcription 3, in patients with advanced metastatic cancer.
Patients and Methods: Patients were treated with TTI-101 orally twice daily in 28-day cycles at four dose levels (DL): 3.2 (DL1), 6.4 (DL2), 12.8 (DL3), and 25.6 (DL4) mg/kg/day (“3+3” design). Three TTI-101 formulations were used in a stepwise manner (NCT03195699).
Results: Sixty-four patients were treated (median age, 63 years; male sex, 52%; median number of prior therapies, 3). No dose-limiting toxicities or fatal treatment-related adverse events (TRAE) were observed. Diarrhea (mostly grade 1/2) was the only TRAE observed in ≥30% of subjects. Five patients experienced grade 3 TRAEs that resolved. TTI-101 showed linear pharmacokinetics from DL1 to DL3, with the pharmacokinetics plateauing at DL3. The recommended phase II dose is 12.8 mg/kg/day (DL3). Of the 41 patients who were evaluable for response, five (12%) had confirmed partial responses (cPR) and 17 (41%) had stable disease. Three (18%) of the 17 patients with hepatocellular carcinoma had a cPR (median time to treatment failure, 10.6 months). Two other cPRs were noted in one patient with ovarian cancer and one patient with gastric cancer.
Conclusions: TTI-101 was well tolerated. cPRs were observed across tumor types. The antitumor activity of TTI-101 monotherapy in patients with advanced, metastatic solid tumors is promising. A phase II study of TTI-101 in hepatocellular carcinoma is currently underway.
Journal: Molecular Cancer Research
Stress and Obesity Signaling Converge on CREB Phosphorylation to Promote Pancreatic Cancer
One of the deadliest types of cancer is pancreatic ductal adenocarcinoma (PDAC). Chronic stress and obesity are recognized as risk factors for PDAC. We hypothesized that the combination of stress and obesity strongly promotes pancreatic cancer development and growth. Here, we show that the stress mediator norepinephrine and the β-adrenergic receptor agonist isoproterenol rapidly stimulate cyclic adenosine monophosphate response element-binding protein (CREB) phosphorylation at Ser133 in human PDAC cells. Exposure to the nonselective β-adrenergic receptor antagonist propranolol or selective antagonists, including nebivolol, atenolol, or ICI118551, blocked CREB phosphorylation elicited by norepinephrine or isoproterenol in PDAC cells. Stimulation of PDAC cells with neurotensin, a neuropeptide implicated in obesity and PDAC, also stimulated CREB phosphorylation at Ser133. Mechanistically, norepinephrine induced CREB phosphorylation at Ser133 via PKA, whereas neurotensin promoted CREB phosphorylation predominantly through protein kinase D. Our results indicate that CREB is a point of signal convergence that mediates proliferation in PDAC cells and raised the possibility that stress and diet cooperate in promoting PDAC in vivo. To test this notion, mice expressing KrasG12D in all pancreatic lineages (KC mice) and fed an obesogenic high fat, calorie diet that promotes early PDAC development were subjected to social isolation stress. We show that social isolation stress induced a significant increase in the proportion of advanced PDAC precursor lesions (pancreatic intraepithelial neoplasia) in KC mice subjected to an obesogenic high fat, calorie diet.
Implications: Our data imply that chronic (social isolation) stress cooperates with diet-induced obesity in accelerating the development of pancreatic cancer.
This article was highlighted in the March issue.
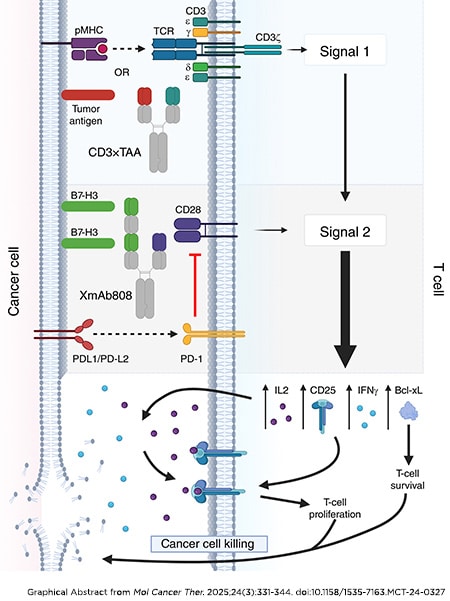
Journal: Molecular Cancer Therapeutics
T-cell activation is a multistep process requiring T-cell receptor engagement by peptide–MHC complexes (Signal 1) coupled with CD28-mediated costimulation (Signal 2). Tumors typically lack expression of CD28 ligands, so tumor-specific Signal 1 (e.g., neoepitope presentation) without costimulation may be ineffective or even induce T-cell anergy. We designed the bispecific antibody XmAb808 to co-engage the tumor-associated antigen B7-H3 with CD28 to promote T-cell costimulation within the tumor microenvironment. XmAb808 costimulation was measured by its ability to activate and expand T cells and enhance T cell–mediated cancer cell killing in cocultures of human peripheral blood mononuclear cells and cancer cells and in mice engrafted with human peripheral blood mononuclear cells and tumor xenografts. XmAb808 avidly bound cancer cells and stimulated IL2 and IFNγ secretion from T cells cocultured with cancer cells engineered to deliver Signal 1 to T cells via a surface-expressed anti-CD3 antibody. XmAb808 enhanced expression of the antiapoptotic factor Bcl-xL and CD25, promoting survival and IL2-dependent expansion of T cells coupled with increased T cell–mediated cytotoxicity in vitro. XmAb808 combined with an EpCAM×CD3 bispecific antibody to enhance target cell killing through IL2-dependent expansion of CD25+ T cells. This combination also suppressed pancreatic tumor xenograft growth in mice. Furthermore, XmAb808 combined with an anti–programmed cell death protein 1 antibody to suppress breast tumor xenograft growth in mice. XmAb808 as monotherapy and in combination with an anti–programmed cell death protein 1 antibody is currently in clinical development in patients with advanced solid tumors. Our results suggest that XmAb808 may also combine with tumor antigen–targeted anti-CD3 (Signal 1) T-cell engagers.
This article was highlighted in the March issue.
Journal: Cancer Research Communications
Variant of Uncertain Significance Patterns among Patients with Early-Onset Colorectal Cancer
The increasing burden of colorectal cancer among adults younger than age 50 years (early-onset colorectal cancer) and new National Comprehensive Cancer Network guidelines recommending universal genetic testing in early-onset colorectal cancer emphasize the need for accurate and timely variant classification in clinical care. In this study, we investigated germline variant of uncertain significance (VUS) patterns among diverse individuals with early-onset colorectal cancer. A total of 3,980 individuals ages 15 to 49 years when diagnosed with a first primary colorectal cancer, including 1,001 cases identifying as non-White, who underwent genetic testing for 14 colorectal cancer susceptibility genes performed by a clinical testing laboratory were included. A five-tier classification system was applied to all alterations to classify VUSs and was updated in March 2024. Disparities in variant reclassification rates emerged by self-identified race/ethnicity (P < 0.0001). A total of 4.8% of Ashkenazi Jewish, 18.2% of Asian, 12.2% of Black, 7.6% of Hispanic, and 6.7% of White individuals had at least one reclassified VUS. After reclassification, 356 individuals (8.9%) presented with 1+ VUSs. VUSs significantly varied by self-identified race/ethnicity (P = 0.008). Young individuals identifying as Black and Hispanic had significantly higher odds of presenting with a VUS compared with those identifying as White in multivariable logistic regression models. Individuals who identified as Black and Asian were significantly more likely to present with a VUS in PMS2 (OR = 3.59; 95% confidence interval, 1.73–7.48; P = 0.0006) and MSH2 (OR = 3.14; 95% confidence interval, 1.17–8.45; P = 0.02), respectively, compared with those identifying as White. These findings define unique VUS patterns in early-onset colorectal cancer by race and ethnicity, pointing to distinct germline variant spectra and to the potential for the discovery of novel ancestry-specific variants associated with early-onset colorectal cancer that will guide efforts to reduce clinical uncertainty and improve equitable care.
Significance: Among individuals with early-onset colorectal cancer, germline VUS reclassification as well as rates of VUSs in cancer susceptibility genes differed by self-identified race/ethnicity. These findings point to the importance of VUS reclassification as this may alter clinical management and to distinct germline variant spectra among diverse patients with early-onset colorectal cancer.