FDA Approvals in Oncology: January-March 2025
To help our readers keep track of the cancer therapies approved by the U.S. Food and Drug Administration (FDA), understand their impact for patients, and put them in context of the current therapeutic landscape, Cancer Research Catalyst provides a quarterly review of the latest approvals in oncology from the FDA.
Kicking off the first quarter of 2025, the FDA issued 13 approvals, covering nine types of cancer and two disorders, for diverse treatments leveraging antibody-based constructs, biomarker-guided patient selection, and particle radiation.
Overall, these approvals illustrate the continued progress with developing treatments that provide superior benefits and broaden treatment options for patients, especially populations with limited therapeutic avenues available. Many of these approvals used biomarkers based on the specific genetic or molecular characteristics of the tumors to match patients with the best treatment options.
Targeting Tumor Drivers: HER2, TROP2, KRAS, and PSMA
Targeting the signaling pathways that drive malignant activity is fundamental to cancer care, and recent approvals show how far these strategies have evolved. Human epidermal growth factor receptor 2 (HER2) is overexpressed on the surface of some types of breast cancer cells, and in 2006 the HER2-targeting trastuzumab (Herceptin) became the first FDA-approved tumor-targeting antibody. Now, multiple therapies built off its framework are helping patients, too.
- Fam-trastuzumab deruxtecan-nxki (T-Dxd; Enhertu) was approved for patients with unresectable or metastatic, hormone receptor (HR)-positive breast cancer with HER2 expression classified as low or ultralow, selected using a companion diagnostic that received concurrent approval. The approval covers patients whose tumors have progressed during or following endocrine therapy.
Whereas HER2 heterogeneity has been associated with resistance to traditional HER2 antibodies, T-Dxd is an antibody-drug conjugate (ADC) that utilizes trastuzumab’s HER2-targeting backbone to deliver a potent chemotherapy payload directly to tumors to enhance their elimination. T-Dxd was previously approved for all unresectable or metastatic, HER2-positive solid cancers, as well as breast cancers with low HER2 expression that were previously treated with chemotherapy. This latest approval, which expands the therapy’s reach to ultralow HER2-expressing tumors, is a promising step forward.
The results from the pivotal trial were presented at the 2024 San Antonio Breast Cancer Symposium (SABCS), an annual conference co-organized by the American Association for Cancer Research (AACR).
Another ADC approval covered patients whose breast cancer lacks HER2, by going after another target.
- Datopotamab deruxtecan-dlnk (dato-DXd; Datroway) was approved for patients with HR-positive, HER2-negative metastatic breast cancer after prior endocrine and chemotherapy treatments.
Dato-Dxd targets TROP-2, a protein frequently overexpressed in aggressive HER2-negative cancers, and delivers the same cytotoxic agent as T-Dxd, providing a crucial new option for challenging-to-treat breast cancer cases. This is the first FDA approval for dato-DXd, which became the second Trop-2-targeting ADC approved for pretreated HR-positive, HER2-negative advanced or metastatic breast cancer. The results from the pivotal trial were presented at SABCS 2023.
After trastuzumab’s initial breakthrough, antibodies were developed to target several other tumor-associated pathways, including epidermal growth factor receptor (EGFR). But only recently have therapies successfully targeted mutant forms of KRAS, which is one of the most notorious oncogenic drivers and was once thought “undruggable.” One approval reflected the effectiveness of targeting KRAS G12C and EGFR together.
- Sotorasib (Lumakras) was approved in combination with panitumumab (Vectibix) for patients with metastatic colorectal cancer harboring the KRAS G12C mutation who were previously treated with chemotherapy. Concurrently, the FDA approved a companion diagnostic to determine the presence of the KRAS G12C mutation.
Sotorasib is a small molecule inhibitor of KRAS G12C activity, and panitumumab is an EGFR inhibitor. This approval, sotorasib’s first in colorectal cancer, follows its earlier accelerated approval for KRAS G12C-mutant non-small cell lung cancer, and is the second approval of a combination targeting KRAS G12C and EGFR for this colorectal cancer population. By combining KRAS and EGFR blockade, this regimen addresses multiple drivers linked to resistance.
In addition to chemotherapy drugs, antibodies can be equipped with other components to kill cancer cells, such as the antibody-based therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto).
- A previous approval for lutetium Lu 177 vipivotide tetraxetan was expanded to include patients with metastatic, castration-resistant prostate cancer that expresses prostate membrane-specific antigen (PSMA), has been treated with androgen receptor pathway inhibitor therapy, and for which taxane-based chemotherapy can be delayed.
This therapy delivers beta-particle radiation selectively to PSMA-expressing tumor cells, and this approval significantly expands the number of prostate cancer patients who can receive a chemotherapy-free alternative.
Hematologic Malignancies
Several first quarter approvals—including another ADC—provide expanded options for patients with hematologic malignancies, including non-Hodgkin lymphomas and leukemia, and a myeloid disorder.
- Brentuximab vedotin (Adcetris) was approved alongside lenalidomide and rituximab for patients with relapsed or refractory large B-cell lymphoma (LBCL) that cannot be treated with stem cell transplant or CAR T-cell therapy.
Brentuximab vedotin is an ADC that targets the CD30 receptor that can be found on the surface of cancerous B cells. Previously approved for other lymphomas and mycosis fungoides, this regimen represents brentuximab vedotin’s first approval for LBCL, and covers diffuse LBCL not otherwise specified, diffuse LBCL arising from indolent lymphoma, and high-grade B-cell lymphoma. Lenalidomide is an immunomodulatory drug often included in B-cell treatment regimens, and rituximab is an antibody that targets the CD20 surface receptor expressed on B cells.
- Acalabrutinib (Calquence) was approved as first-line therapy in combination with bendamustine and rituximab, and as second-line monotherapy, for patients with mantle cell lymphoma (MCL).
Acalabrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK), and this is its first full approval for previously untreated MCL. Acalabrutinib is a targeted therapy designed to suppress the BTK signals that help MCL cells proliferate, and it previously received accelerated approval as a first-line therapeutic option for these patients. Bendamustine is a type of chemotherapy often used to treat certain leukemias, lymphomas, and myelomas.
This quarter also saw a new approval for patients preparing to receive a hematopoietic stem cell transplantation (HSCT).
- Treosulfan (Grafapex) was approved in combination with fludarabine as a conditioning regimen prior to allogeneic HSCT for patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The approval covers patients 1 year and older.
This is the first FDA approval for treosulfan, which helps clear diseased bone marrow stem cells by disrupting DNA replication and cell division, so that the donor stem cells can properly engraft and reconstitute blood cell populations in the patient.
Immunotherapy Updates
The same principles that guide patient selection for molecularly targeted therapies are also being applied to immune checkpoint inhibitors (ICIs) that target programmed cell death protein 1 (PD-1) receptor and its ligand, PD-L1, to enhance immune cells’ ability to attack and eliminate tumors. Notably, all three approvals of this class of treatment cover newly diagnosed, untreated patients.
- Pembrolizumab (Keytruda), combined with trastuzumab and chemotherapy, was approved as first-line therapy for patients with HER2-positive, PD-L1-expressing gastric cancers and gastroesophageal junction (GEJ) adenocarcinomas. This indication was previously covered by an accelerated approval for pembrolizumab.
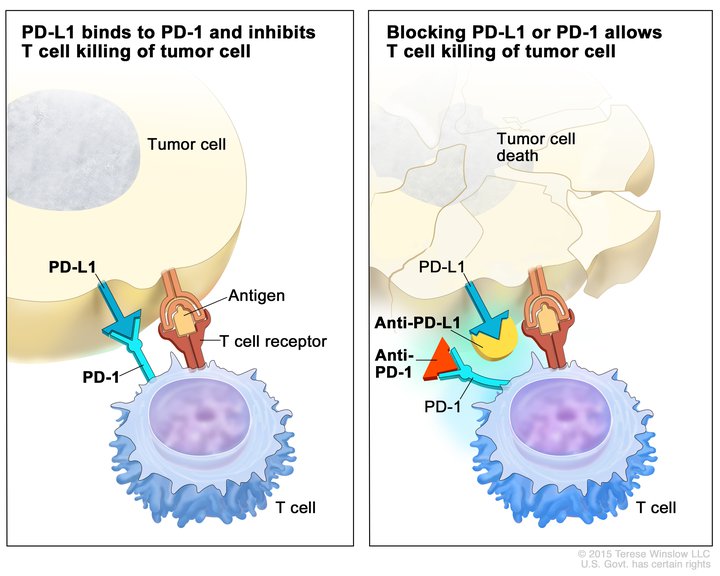
This dual-biomarker strategy aids the patients most likely to benefit from each component, optimizing treatment efficacy.
- Similarly, tislelizumab-jsgr (Tevimbra) was approved with chemotherapy for first-line treatment of unresectable or metastatic esophageal squamous cell carcinoma (ESCC) expressing PD-L1.
- Further broadening immunotherapy applications, durvalumab (Imfinzi) was approved for muscle invasive bladder cancer in combination with chemotherapy, followed by monotherapy post-surgery.
Rare Cancers and Disorders
Three approvals this quarter improved treatment options for certain rare cancers and a genetic disorder, addressing critical unmet needs.
- Mirdametinib (Gomekli) was approved for patients with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas but are unable to undergo full resection.
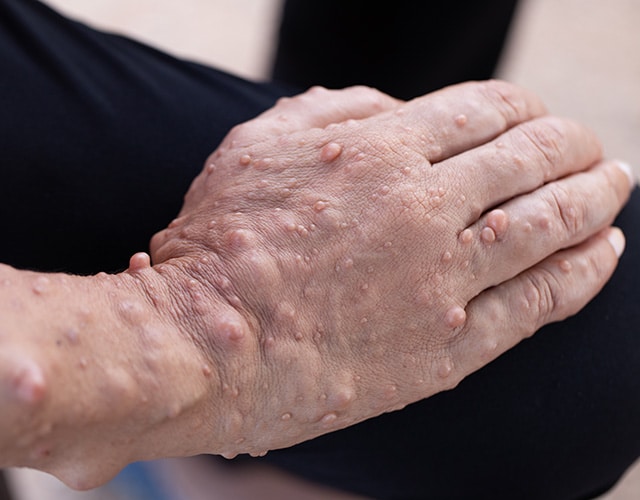
NF1 is a genetic disorder in which a patient’s cells produce a faulty version of the protein neurofibromin that normally regulates cell growth. Mirdametinib, a new inhibitor of MEK1/2, whose aberrant signaling can promote tumor growth, possesses potentially superior blood-brain-barrier penetration. Its first FDA approval provides an important nonsurgical option for NF1 patients.
- Vimseltinib (Romvimza), a CSF1 receptor inhibitor, was approved for patients with symptomatic tenosynovial giant cell tumors (TGCT) that cannot be surgically removed.
This small molecule targeted therapy inhibits the activity of colony-stimulating factor 1 receptor (CSF1R), whose overactivation can drive TGCT development. Vimseltinib’s approval is especially significant given the risks of liver toxicity associated with the only other FDA-approved CSF1R inhibitor for TGCT patients. Results from an earlier clinical trial demonstrating vimseltinib’s benefits were published in the AACR journal Clinical Cancer Research.
- Cabozantinib (Cabometyx) was approved for certain patients with advanced and/or previously treated pancreatic and extra-pancreatic neuroendocrine tumors.
Cabozantinib is a small molecule targeted therapy with inhibitory activity against multiple kinases, including the angiogenesis-promoting vascular endothelial growth factor (VEGF).
Collectively, these FDA approvals in the first quarter of 2025 highlight the strides the field has made in biomarker-guided approaches that expand the therapeutic landscapes for patients with diverse cancers and rare diseases.

