
AACR Virtual Annual Meeting I: Results on a Targeted Therapeutic for Lung Cancer Support FDA Approval
Over the course of the two-day AACR Virtual Annual Meeting I, more than 61,000 people from 140 countries around the...


Over the course of the two-day AACR Virtual Annual Meeting I, more than 61,000 people from 140 countries around the...

A short time after Mallika Lala, PhD, finished her presentation titled “Pembrolizumab 400 mg Q6W dosing: First clinical outcomes...

The cutting-edge scientific program for the AACR Virtual Annual Meeting I began Monday, April 27, with an Opening Clinical Plenary featuring...
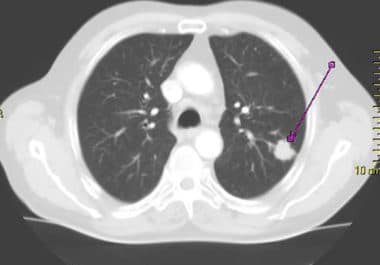
Screening for lung cancer using low-dose computed tomography (CT) was introduced in the United States in 2013. Recent data...
The first new anticancer therapeutics approved by the U.S. Food and Drug Administration (FDA) in 2020 are for the...
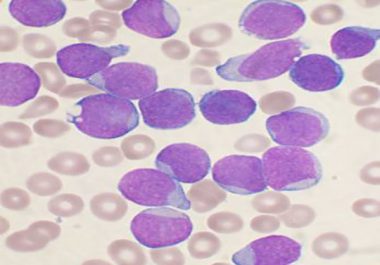
The first paper to be accepted by the AACR’s latest journal, Blood Cancer Discovery, was published online last week....

The U.S. Food and Drug Administration (FDA) approved a new molecularly targeted therapeutic called fam-trastuzumab deruxtecan-nxki (Enhertu) for treating...
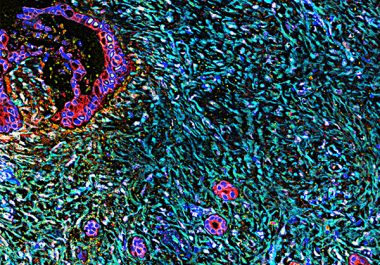
In the final days of 2019, the U.S. Food and Drug Administration (FDA) expanded the use of a previously...

In November and the first half of December, the U.S. Food and Drug Administration (FDA) announced three approvals for...

On Wednesday, the U.S. Food and Drug Administration (FDA) expanded the use of the molecularly targeted therapeutic niraparib (Zejula)...