
STAR Act Aims to Boost Pediatric Research
This week, President Trump signed the Childhood Cancer Survivorship, Treatment, Access and Research (STAR) Act, which is aimed at...


This week, President Trump signed the Childhood Cancer Survivorship, Treatment, Access and Research (STAR) Act, which is aimed at...
“We are in a promising time for cancer research and treatment; however, ovarian cancer remains difficult to treat, since...

Skin cancer is the most commonly diagnosed cancer in the United States. Every year, about 5 million Americans are...

On May 4, the U.S. Food and Drug Administration (FDA) approved a combination of molecularly targeted therapeutics for the...

Norman E. “Ned” Sharpless, MD, who became director of the National Cancer Institute in October 2017, recently unveiled areas...

Earlier this week, the U.S. Food and Drug Administration (FDA) approved expanding the use of the immunotherapeutic tisagenlecleucel (Kymriah)...

The AACR Annual Meeting 2018 drew more than 22,600 people to Chicago, providing a front-row seat to some exciting...

Early this week, the U.S. Food and Drug Administration (FDA) approved a combination immunotherapy regimen for treating certain patients...

This post originally appeared on the Cancer Today website. Roads and roadblocks, both literal and metaphorical, figured into a...
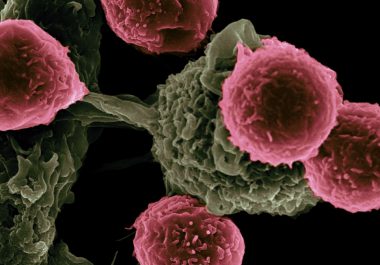
An exciting area in drug development, immunotherapy is being increasingly utilized by patients with different cancer types. These treatments...