
Senate Forum Examined the Ramifications of NIH Funding Cuts
A congressional forum hosted by Senators Tammy Baldwin and Peter Welch examined how NIH funding cuts could impact cancer...


A congressional forum hosted by Senators Tammy Baldwin and Peter Welch examined how NIH funding cuts could impact cancer...

In the first quarter of 2025, the FDA issued 13 approvals for oncology treatments, covering nine types of cancer...

Natalie Snider-Hoy, a PhD candidate at Wayne State University, attended AACR Early-career Hill Day 2025 to advocate for sustained...

In the final three months of 2024, the FDA issued 15 approvals in oncology, including several therapies that are...

Ten-year-old Michael Methner told his story about his experience being diagnosed with optic nerve glioma at the Rally for...

This quarter’s 16 approvals include a first-in-class cell therapeutic, a flurry of new options for EGFR-mutated lung cancer, and...

Expanding clinical trial eligibility requirements can help increase participation and more accurately show how treatments work in a real-world...
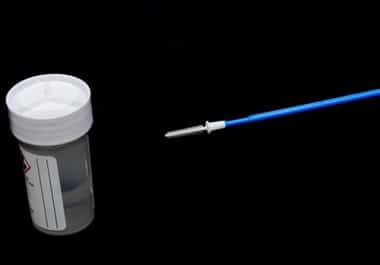
New cervical cancer self-screening options and a portable blood test device could lead to changes in at-home cancer screening...

Highlights from a workshop and journal article that shared key considerations for including overall survival as an endpoint in...

The FDA issued 18 oncology-related approvals in Q2 2024 including two first-in-class drugs and a bispecific T-cell engager for...