
FDA Approvals in Oncology: January-March 2025
In the first quarter of 2025, the FDA issued 13 approvals for oncology treatments, covering nine types of cancer...


In the first quarter of 2025, the FDA issued 13 approvals for oncology treatments, covering nine types of cancer...

In the final three months of 2024, the FDA issued 15 approvals in oncology, including several therapies that are...

This quarter’s 16 approvals include a first-in-class cell therapeutic, a flurry of new options for EGFR-mutated lung cancer, and...
New cervical cancer self-screening options and a portable blood test device could lead to changes in at-home cancer screening...

The FDA issued 18 oncology-related approvals in Q2 2024 including two first-in-class drugs and a bispecific T-cell engager for...

The FDA issued 14 oncology approvals this quarter, including the first tumor-infiltrating lymphocyte therapy.

In the final quarter of 2023, the FDA issued 17 approvals specifically for the treatment of tumors.
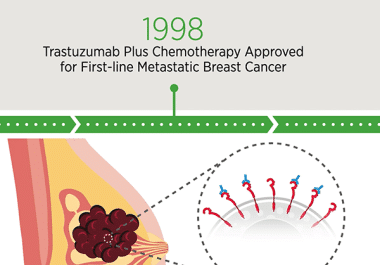
The AACR Annual Meeting 2023, held April 14-19, commemorated several milestones, including the 25th anniversary of the approval of...

FDA approvals issued in third quarter 2023 included a new treatment for prostate cancer harboring BRCA mutations, two new...