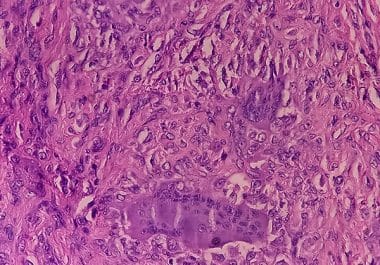
CSF1 Receptor Inhibitor Vimseltinib Approved for Tenosynovial Giant Cell Tumor
The FDA has approved a CSF1 small molecule inhibitor for certain patients with symptomatic tenosynovial giant cell tumor. The...


The FDA has approved a CSF1 small molecule inhibitor for certain patients with symptomatic tenosynovial giant cell tumor. The...
The FDA has approved the antibody-drug conjugate brentuximab vedotin, plus lenalidomide and rituximab, for certain cases of pretreated large...
The FDA has approved a new MEK inhibitor for certain patients with symptomatic neurofibromatosis type 1. The U.S. Food...
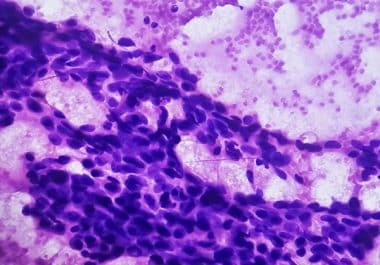
The FDA has approved fam-trastuzumab deruxtecan-nxki for certain HER2-low or HER2-ultralow breast cancers. The U.S. Food and Drug Administration...

The FDA approved treosulfan and fludarabin for patients with hematologic disorders prior to stem cell transplantation. The U.S....
The FDA has issued its first approval for datopotamab deruxtecan-dlnk, a targeted therapy, for certain types of breast cancer. ...

The FDA approved sotorasib and panitumumab for metastatic colorectal cancer with the KRAS G12C mutation. The U.S. Food and...
Acalabrutinib was granted traditional approval as a monotherapy for previously treated MCL and as part of a combination for...

The FDA approved nivolumab and hyaluronidase-nvhy as an injectable therapy for multiple tumor types. The U.S. Food and Drug...

The FDA approved the kinase inhibitors encorafenib and cetuximab plus chemotherapy for the treatment of certain colorectal cancers. The...