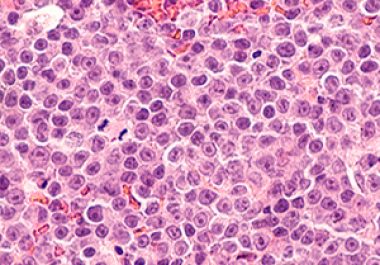
New Approval Adds to Growing List of Therapies for B-cell Lymphoma
The FDA granted accelerated approval to another bispecific T-cell engager to treat certain B-cell lymphomas The U.S. Food and...


The FDA granted accelerated approval to another bispecific T-cell engager to treat certain B-cell lymphomas The U.S. Food and...
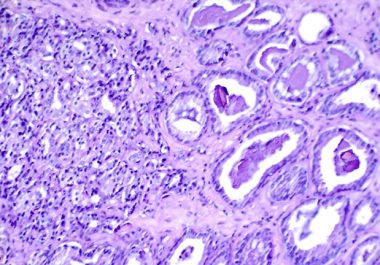
The FDA approved the DNA damage repair inhibitor olaparib, in combination with other therapies, for Castration-resistant or refractory prostate...
The FDA granted accelerated approval to the first bispecific T-cell engager to treat two types of B-cell lymphomas The...
The FDA has approved a new antibody-drug conjugate to treat two types of non-Hodgkin B-cell lymphomas The U.S. Food...
This is the first modified, donor cord blood–based stem cell therapy approved to reduce time to hematopoietic recovery and...
A targeted therapy is now available for more patients with advanced bladder cancers The U.S. Food and Drug Administration...
The FDA has approved the monoclonal antibody retifanlimab-dlwr for some patients with Merkel cell carcinoma. The U.S. Food and...
A targeted therapy regimen was approved to treat some children with brain cancer The U.S. Food and Drug Administration...
The FDA approved abemaciclib with endocrine therapy for HR-positive, HER2-negative, early-stage breast cancer at high risk of recurrence. The...

A drug that selectively targets cancer cells was approved for the treatment of certain HR-positive breast cancers. The U.S....