New Targeted Therapy for Acute Myeloid Leukemia
A second inhibitor of mutant IDH1 was approved to treat certain blood cancers. The U.S. Food and Drug Administration...

A second inhibitor of mutant IDH1 was approved to treat certain blood cancers. The U.S. Food and Drug Administration...

A targeted therapy was approved for a subset of treatment-resistant ovarian, fallopian tube, and peritoneal cancers. The U.S. Food...
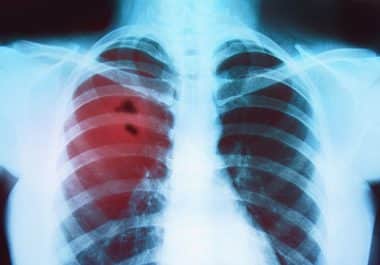
The FDA approved the immune checkpoint inhibitor tremelimumab to treat certain patients with non-small cell lung cancer. The U.S....

The CD30-targeted antibody-drug conjugate was approved for some children with blood cancer. The U.S. Food and Drug Administration (FDA)...
The immune checkpoint inhibitor cemiplimab-rwlc was approved in combination with chemotherapy for certain patients with non-small cell lung cancer. ...
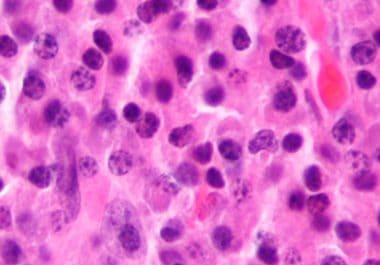
The FDA granted accelerated approval to a targeted immunotherapy for patients with relapsed or refractory multiple myeloma. The U.S....
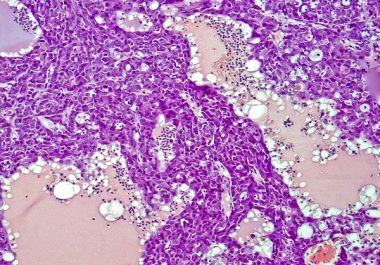
The FDA approved the combination of two checkpoint inhibitors for certain patients with hepatocellular carcinoma. The U.S. Food and...
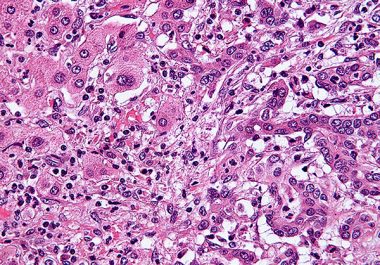
The FDA granted accelerated approval to a new targeted therapy for certain patients with advanced or metastatic cholangiocarcinoma. The...
The FDA granted accelerated approval to selpercatinib to treat solid tumors harboring RET gene fusions. The U.S. Food and...

The FDA granted full approval to selpercatinib for the treatment of patients with advanced lung cancer. The U.S. Food...