Prolonging Disease-free Survival from Esophageal and Gastroesophageal Junction Cancer
The FDA has approved an immunotherapeutic for certain patients with these cancers who had their cancer completely resected and...


The FDA has approved an immunotherapeutic for certain patients with these cancers who had their cancer completely resected and...
The FDA has granted accelerated approval to an immunotherapeutic to be used in combination with a targeted therapeutic and...
The FDA has approved the targeted therapeutic loncastuximab tesirine-lpyl to treat certain adult patients with large B-cell lymphoma. The...
The FDA has granted accelerated approval to an immunotherapy to treat certain adult patients with endometrial cancer that tests...

The FDA has approved an immunotherapeutic in combination with chemotherapy to treat certain patients with gastric cancer, gastroesophageal junction...
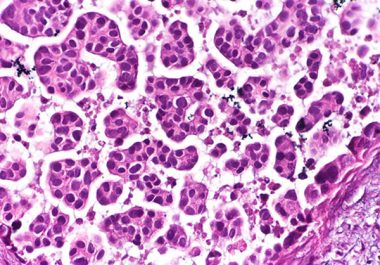
The FDA granted accelerated approval to the targeted therapeutic sacituzumab govitecan to treat certain patients with the most common...
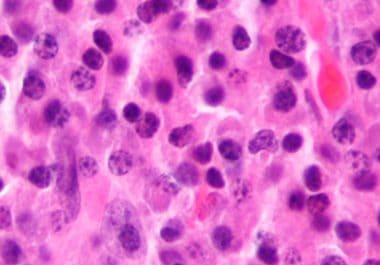
The FDA approved the first CAR T-cell therapy to treat certain adult patients with multiple myeloma. The U.S. Food...
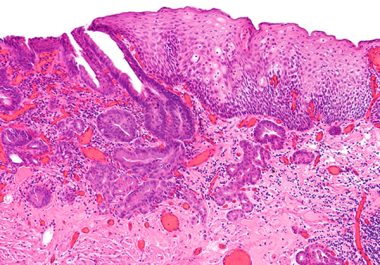
the immunotherapeutic pembrolizumab in combination with chemotherapy WAS APPROVED to treat certain adult patients with esophageal cancer. The U.S....
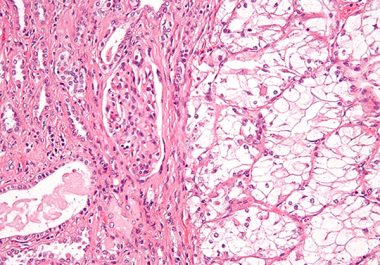
The FDA approved the molecularly targeted therapeutic tivozanib to treat certain adult patients with the most common type of...
The FDA granted accelerated approval to a CAR T-cell therapy for certain adult patients with follicular lymphoma, a common...