
First-in-class Cell Therapy for Steroid-refractory Graft Versus Host Disease
The FDA approved the mesenchymal stromal cell therapy remestemcel-L-rknd for some pediatric patients as young as 2 months. ...


The FDA approved the mesenchymal stromal cell therapy remestemcel-L-rknd for some pediatric patients as young as 2 months. ...
The FDA approved ensartinib for certain patients with locally advanced or metastatic non-small cell lung cancer. The U.S. Food...
The FDA approved cosibelimab-ipdl for certain skin cancers that cannot be treated with surgery or radiation. The U.S. Food...
The FDA approved durvalumab for some patients with previously treated limited-stage small cell lung cancer. The U.S. Food...
The FDA approved the targeted therapy zenocutuzumab-zbco for certain patients with lung or pancreatic cancer. The U.S. Food and...
The FDA granted accelerated approval to the first-in-class HER2-directed bispecific antibody zanidatamab-hrii. The U.S. Food and Drug Administration (FDA)...

The FDA Project Renewal initiative has updated the approved indications for fludarabine phosphate chemotherapy in chronic lymphocytic leukemia. The...
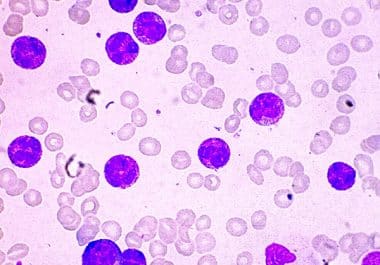
The FDA approved revumenib, a first-in-class menin inhibitor, to treat acute leukemias with a KMT2A translocation. The U.S. Food...
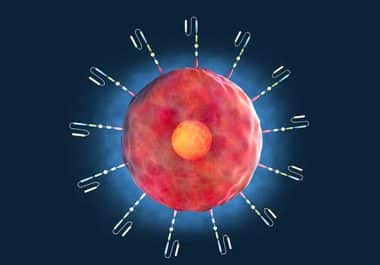
The cell-based immunotherapy was approved for adults with relapsed or refractory acute lymphoblastic leukemia. The U.S. Food and Drug...
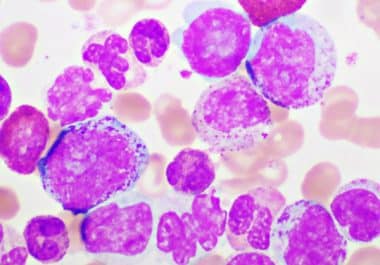
The therapeutic was approved for certain patients with Philadelphia chromosome-positive chronic myeloid leukemia. The U.S. Food and Drug Administration...