Immunotherapy for Patients With a Rare Form of Non-Hodgkin Lymphoma
The FDA has approved a CAR T-cell immunotherapy to treat certain adult patients with mantle cell lymphoma. The U.S....


The FDA has approved a CAR T-cell immunotherapy to treat certain adult patients with mantle cell lymphoma. The U.S....
The FDA approved an oral combination of an epigenetic therapy along with an enzyme inhibitor to treat certain adults...
The FDA approved the use of an immune checkpoint inhibitor as a first-line treatment for patients with colorectal cancers...
The FDA approved the use of an immune checkpoint inhibitor to treat certain patients with cutaneous squamous cell carcinoma. ...
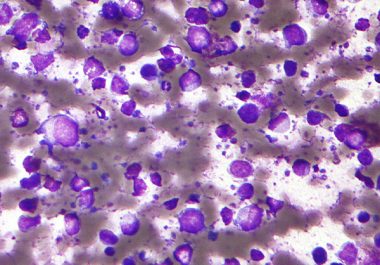
The FDA has approved a targeted therapy drug to treat certain adult patients with diffuse large B-cell lymphoma. The...
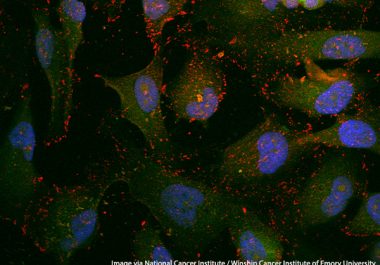
The FDA approved the use of a companion diagnostic to identify patients with tumors that have large numbers of...
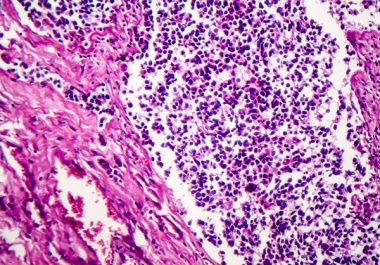
The FDA approved an analog of a marine compound to treat certain patients with small cell lung cancer. The...
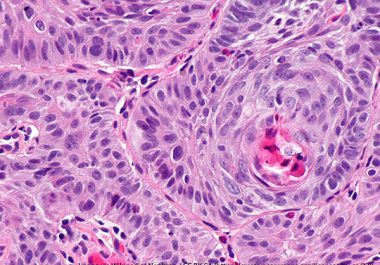
The FDA approved the use of an immune checkpoint inhibitor to treat certain patients with a common form of...
The FDA approved a combination of an immunotherapy and a therapeutic that can stop tumors from growing blood vessels...
The FDA has expanded the use of the PARP-targeted therapeutic olaparib to include the treatment of certain patients with...