Targeting Several Blood Cancers
The FDA has approved a new molecularly targeted therapeutic called duvelisib for treating certain patients with blood cancer. The...

The FDA has approved a new molecularly targeted therapeutic called duvelisib for treating certain patients with blood cancer. The...
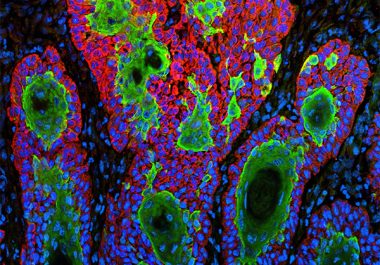
The FDA decision makes cemiplimab-rwlc the first-ever treatment approved specifically for treating squamous cell carcinoma. The U.S. Food and...

The FDA has expanded the use of the immunotherapeutic nivolumab to treat certain patients with small cell lung cancer....
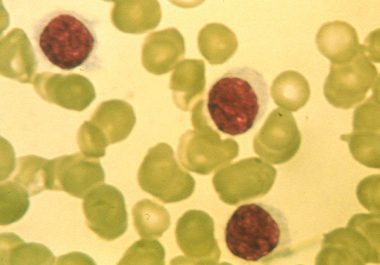
The FDA has approved the first of a new type of molecularly targeted therapy for use in treating certain...
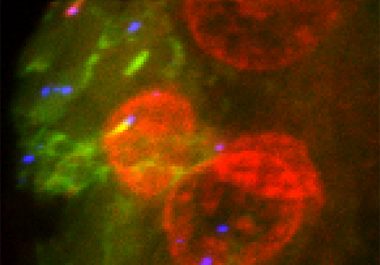
The FDA has approved expanding the use of lenvatinib to treat certain patients with the most common type of...
The FDA has approved the combination of ibrutinib and rituximab as a new treatment for a rare form of...

The FDA has approved a new targeted radiotherapeutic for treating two rare neuroendocrine tumors, pheochromocytomas and paragangliomas. The U.S....
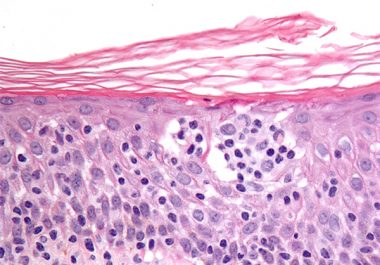
The FDA has approved a new immunotherapeutic for treating two types of cutaneous T-cell lymphoma, mycosis fungoides and Sézary...
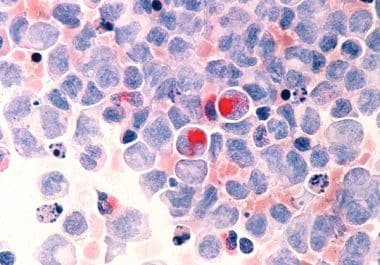
The new therapeutic was approved by the FDA for treating patients whose leukemia tests positive for a mutation in...
The combination of ipilimumab and nivolumab was approved by the FDA for metastatic colorectal cancer that is positive for...