First Targeted Therapeutic for BRCA-mutant Breast Cancer
The FDA has approved the molecularly targeted therapeutic olaparib for treating patients who have HER2-negative breast cancer and who...


The FDA has approved the molecularly targeted therapeutic olaparib for treating patients who have HER2-negative breast cancer and who...
The FDA has expanded the use of a CD30-targeted antibody?drug conjugate to include two additional types of lymphoma. The...
The newly approved therapeutic is for certain patients with an aggressive type of non-Hodgkin lymphoma called mantle cell lymphoma....
The second of a groundbreaking new type of immunotherapy called CAR T-cell therapy is approved for certain types of...
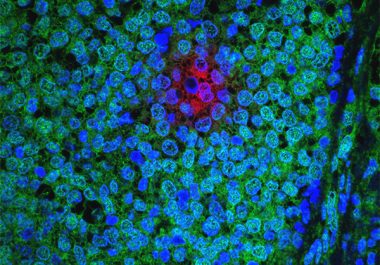
The FDA has approved a new molecularly targeted therapeutic to treat breast cancer patients with a specific subtype of...
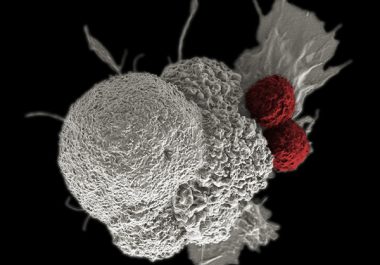
The FDA decision means the immune checkpoint inhibitor nivolumab is now approved for eight types of cancer. Nivolumab (Opdivo)...
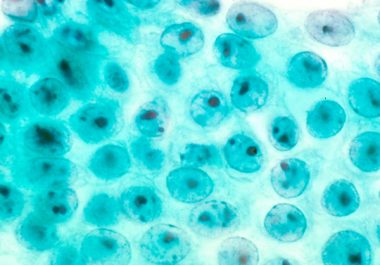
The FDA has approved pembrolizumab for treating certain patients with advanced stomach (gastric) cancer. Use of the immunotherapeutic pembrolizumab...
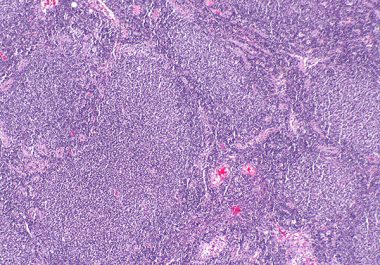
A new molecularly targeted therapeutic to treat certain patients with follicular lymphoma, a common type of non-Hodgkin lymphoma, has...
The FDA has approved a CD22-targeted antibody-drug conjugate for treating certain adults with ALL. The U.S. Food and Drug...
The new treatment, Vyxeos, comprises a combination of two commonly used cytotoxic chemotherapeutics inside a nanosized particle. The U.S....