
Liquid Biopsy Approved for Lung Cancer
The FDA-approved diagnostic test uses blood samples to look for specific mutations in certain patients with metastatic lung cancer....


The FDA-approved diagnostic test uses blood samples to look for specific mutations in certain patients with metastatic lung cancer....
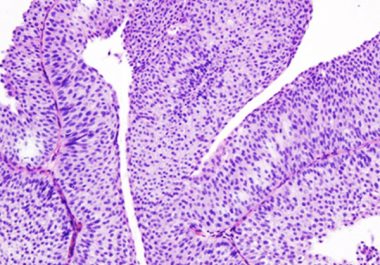
The approval provides the first new treatment option in more than 30 years for patients with the most common...
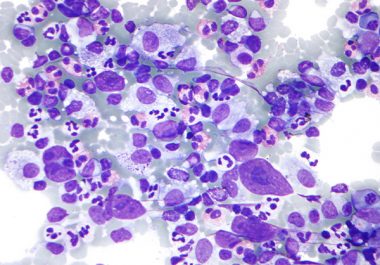
The U.S. Food and Drug Administration approval provides a new option for patients with the most common type of...
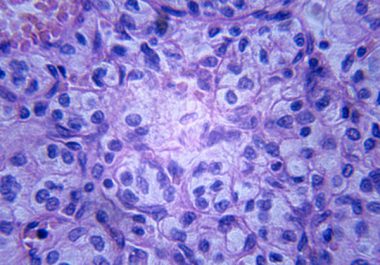
The U.S. Food and Drug Administration approval provides a new option for patients with the most common type of...

The U.S. Food and Drug Administration approval increases the number of patients with lung cancer for whom afatinib (Gilotrif)...
The newly approved therapeutic improved survival for patients with the most common type of kidney cancer compared with standard...
Test uses blood samples, providing an alternative method for colorectal cancer screening The U.S. Food and Drug Administration (FDA)...
The U.S. Food and Drug Administration approval provides a new treatment option for certain patients with chronic lymphocytic leukemia....

Targeted therapeutic is the first treatment approved by the FDA for patients with lung cancer positive for mutations in...
The U.S. Food and Drug Administration (FDA) approval provides a new option for patients with a common type of...