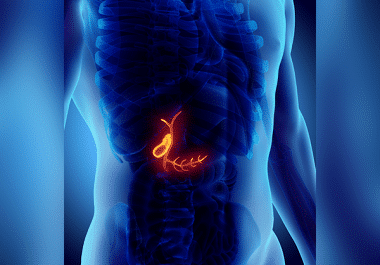
Immunotherapy for Biliary Tract Cancers
The FDA has approved the immune checkpoint inhibitor pembrolizumab for certain advanced cancers of the biliary tract. The U.S....


The FDA has approved the immune checkpoint inhibitor pembrolizumab for certain advanced cancers of the biliary tract. The U.S....
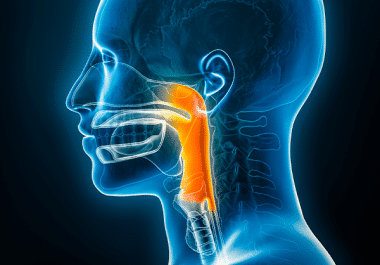
The FDA has approved the immune checkpoint inhibitor toripalimab for some patients with nasopharyngeal carcinoma. The U.S. Food and...
The FDA has approved the IDH1 inhibitor ivosidenib for a group of blood cancers harboring an IDH1 mutation. The...

The FDA has approved the inhibitor entrectinib for some patients 1 month of age or older with solid tumors...
The FDA has approved pembrolizumab for the treatment of certain surgically removable tumors before and after resection. The U.S....
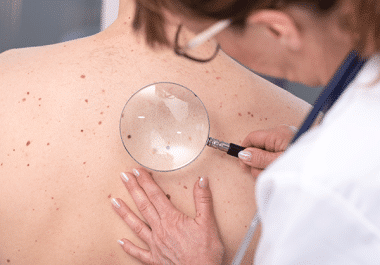
The FDA approved nivolumab for some patients with melanoma whose tumor was completely removed through surgery and has not...
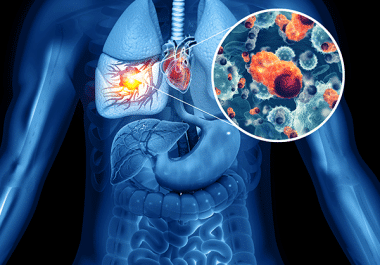
The FDA approved encorafenib with binimetinib as a treatment for certain patients with non-small cell lung cancer. The U.S....
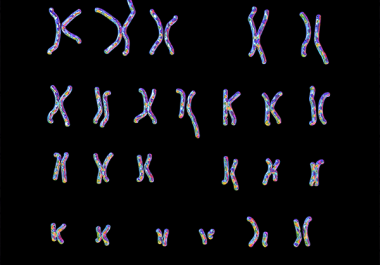
A targeted therapy previously approved for adults is now available for certain pediatric patients with blood cancer. The U.S....
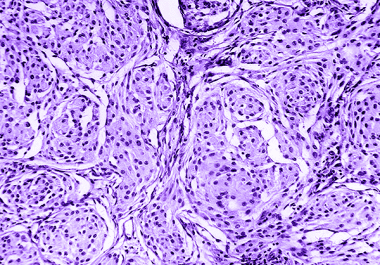
The FDA Project Renewal initiative has updated the approved indications for temozolomide chemotherapy in anaplastic astrocytoma. The U.S. Food...

The FDA approved a kit that allows for liver-directed delivery of the chemotherapy melphalan to treat metastatic liver disease...