New Perspectives on the Risk of Secondary Cancers After CAR T-cell Therapy
Amidst an FDA investigation into secondary cancers after CAR T therapy, researchers show the risk is likely minimal.


Amidst an FDA investigation into secondary cancers after CAR T therapy, researchers show the risk is likely minimal.

Expanding clinical trial eligibility requirements can help increase participation and more accurately show how treatments work in a real-world...

Highlights from a workshop and journal article that shared key considerations for including overall survival as an endpoint in...

The FDA issued 18 oncology-related approvals in Q2 2024 including two first-in-class drugs and a bispecific T-cell engager for...
Dr. Edward Cliff presented a poster on the clinical benefit of cancer drugs granted accelerated approval.

The FDA issued 14 oncology approvals this quarter, including the first tumor-infiltrating lymphocyte therapy.

Patricia M. LoRusso and Stacy Shord discuss dosage optimization's current state and how the FDA-AACR workshop will move the...
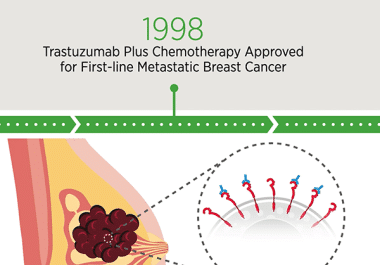
The AACR Annual Meeting 2023, held April 14-19, commemorated several milestones, including the 25th anniversary of the approval of...

With the approval of new anticancer therapeutics, more treatment options become available for patients. Some therapies are new to...

The American Association for Cancer Research (AACR) released its annual Cancer Progress Report today. The report serves as an important record of the...