An Immunotherapy to Target Endometrial Cancer
The FDA has granted accelerated approval to an immunotherapy to treat certain adult patients with endometrial cancer that tests positive for a specific biomarker.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to dostarlimab-gxly (Jemperli) for adult patients with mismatch repair deficient recurrent or advanced endometrial cancer. The immunotherapeutic is indicated for patients with cancer that tests positive for a genetic abnormality that affects the DNA repair process of cells and whose disease has progressed on or following treatment with a platinum-containing chemotherapy.
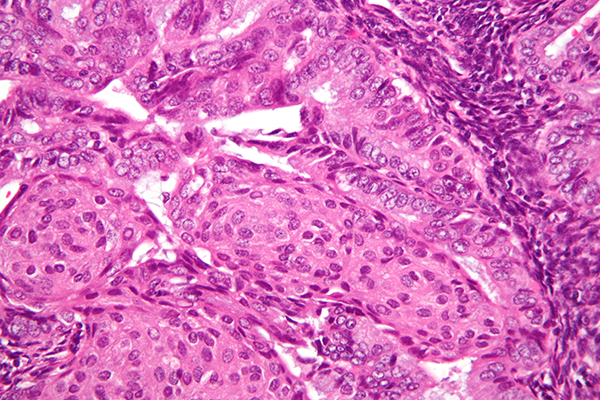
Dostarlimab-gxly targets certain proteins found on cancer cells as well as proteins on T cells to block the action of what is known as the PD-1/PD-L1 pathway, allowing the body’s immune system to attack the cancer.
Data to evaluate the efficacy of dostarlimab-gxly was collected from 71 patients involved in a multicenter, multicohort, open-label trial. The overall response rate was 42.3 percent, with 12.7 percent of study participants showing a complete response and 29.6 percent demonstrating a partial response. The median duration of response was not reached, but 93.3 percent of the patients had a response duration of six months or longer.
Endometrial cancer is a disease in which malignant cells form in the tissues of the endometrium—the lining of the uterus. Uterine cancer is the most common cancer of the female reproductive system diagnosed in the U.S. According to federal statistics, it was estimated that there would be over 66,000 new cases in 2021.
Dostarlimab-gxly was granted accelerated approval by the FDA on April 22, 2021. Accelerated approval means continued approval may be contingent upon a confirmatory trial.
