Drugging an “Undruggable” Protein
A second inhibitor of the difficult-to-target KRAS protein was approved to treat certain lung cancers.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to adagrasib (Krazati) for adult patients with locally advanced or metastatic non-small cell lung cancers (NSCLC) harboring a KRAS G12C mutation who have received at least one prior systemic therapy.
The QIAGEN therascreen KRAS RCQ PCR kit and the Agilent Resolution ctDx FIRST Assay were also approved as companion diagnostic tests to detect the KRAS mutation in tumor tissue and plasma, respectively.
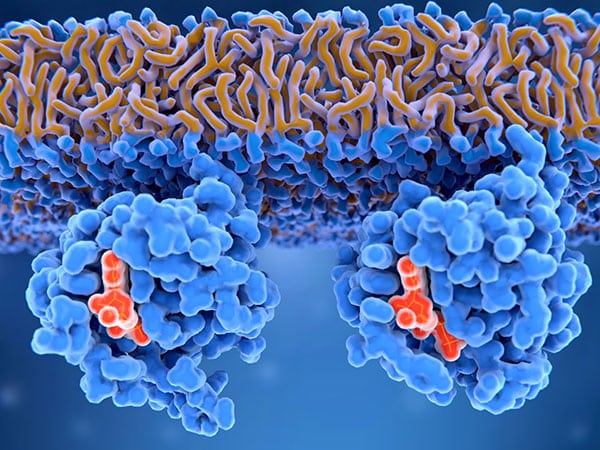
KRAS is a signaling protein that regulates cellular proliferation. When mutated, it can lead to unregulated cellular growth and tumor development. Scientists have long tried to therapeutically inhibit the cancer-promoting activity of mutant KRAS, but the structure of the protein has made it difficult to design effective drugs, leading many to deem KRAS an “undruggable” protein. Adagrasib binds and inhibits the KRAS G12C mutant, one of the most common KRAS mutants. It is the second KRAS inhibitor to be granted accelerated approval by the FDA, after sotorasib in 2021.
The approval is based on results from KRYSTAL-1, a multicenter, single-arm, open-label phase I/II clinical trial that evaluated adagrasib in 112 patients with locally advanced or metastatic NSCLC with KRAS G12C mutations whose disease progressed on or after prior therapy, including platinum chemotherapy and an immune checkpoint inhibitor. Responses were observed in 43 percent of patients, and the median duration of response was 8.5 months.
NSCLC is the most common type of lung cancer. For 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from this disease in the United States.
The FDA rendered its decision on December 12, 2022. Accelerated approval means that continued approval may be contingent upon a confirmatory trial.