First Systemic Therapy Approved for Lung and Pancreatic Cancers With NRG1 Gene Fusions
The FDA approved the targeted therapy zenocutuzumab-zbco for certain patients with lung or pancreatic cancer.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to zenocutuzumab-zbco (Bizengri) for the treatment of adult patients with advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) or pancreatic adenocarcinoma that harbors a gene fusion involving neuregulin 1 (NRG1). The drug is approved for use following disease progression during or after prior systemic therapy.
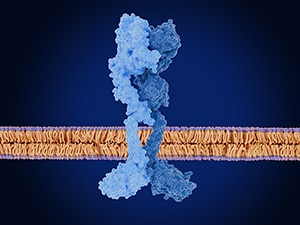
Zenocutuzumab-zbco is a type of targeted therapy called a bispecific antibody. It simultaneously binds to a pair of growth factor receptors called HER2 and HER3, which typically form a complex that can promote cell growth. NRG1 can interact with HER3 to promote the formation of this signaling complex, and NRG1 fusion proteins can cause excessive signaling, which can drive cancer. Zenocutuzumab-zbco blocks HER3 from binding to both HER2 and NRG1. Preclinical data showing the antitumor activity of zenocutuzumab, as well as early clinical data, were reported in the American Association for Cancer Research (AACR) journal Cancer Discovery in 2022.
This is the first FDA approval for zenocutuzumab-zbco, which is the first HER2/HER3-targeting bispecific antibody and the first targeted therapy for lung or pancreatic tumors with NRG1 gene fusions to be approved by the FDA.
The approval was based on results from the multicenter, open-label, multicohort phase II eNRGy clinical trial. The trial enrolled patients with advanced or metastatic NRG1 fusion-positive NSCLC (64 patients) or pancreatic adenocarcinoma (30 patients) who had experienced disease progression on other therapies and treated them with zenocutuzumab-zbco.
Overall, 33% of patients with NSCLC and 40% of patients with pancreatic adenocarcinoma experienced a response. Among these patients, responses lasted a median of 7.4 months for patients with NSCLC and between 3.7 and 16.6 months for patients with pancreatic adenocarcinoma.
The prescribing information for zenocutuzumab-zbco contains a boxed warning for embryo-fetal toxicity.
The recommended dose of zenocutuzumab-zbco is 750 mg given as an intravenous infusion every two weeks until disease progression or unacceptable toxicity.
NSCLC and pancreatic adenocarcinoma are the most common types of lung and pancreatic cancer, respectively. In one study, NRG1 fusions were found to occur in around 0.3% of NSCLCs and 0.5% of pancreatic cancers. According to federal statistics, it was estimated that lung cancer would account for 234,580 cancer cases and 125,070 deaths, and pancreatic cancer would account for 66,440 cases and 51,750 deaths, in the United States in 2024.
The FDA rendered its decision on December 4, 2024. Accelerated approval means that continued approval may be contingent upon a confirmatory trial. Please check this FDA web page for information about any accelerated approvals in oncology that may have been subsequently withdrawn and are no longer FDA-approved. Check this resource for updated information on all therapeutics regulated by the FDA.
