First Targeted Radiotherapy for Certain Pediatric Neuroendocrine Tumors
The FDA has approved lutetium Lu 177 dotatate for children 12 years or older with some gastroenteropancreatic neuroendocrine tumors.
The U.S. Food and Drug Administration (FDA) has approved lutetium Lu 177 dotatate (Lutathera) for the treatment of pediatric patients aged 12 or older with gastroenteropancreatic neuroendocrine tumors (GEP-NETs)—including those in the foregut, midgut, and hindgut—that express the somatostatin receptor (SSTR).
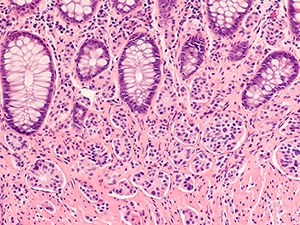
Lutetium Lu 177 dotatate is a radioactive drug. Radioactive lutetium is bound to a compound that targets SSTR, a protein that is highly expressed on the surface of GEP-NET cells. Upon binding to SSTR, lutetium Lu 177 dotatate enters the cell, and the radiation causes cell damage that ultimately leads to cell death.
The drug was previously approved to treat adult patients with SSTR-positive GEP-NETs. The current approval extends the indication to include pediatric patients 12 years of age or older.
The approval was partially based on data from the international, multicenter, open-label, single-arm, phase II NETTER-P clinical trial, which tested lutetium Lu 177 dotatate in four adolescent patients with locally advanced inoperable or metastatic SSTR-positive GEP-NETs. The approval was also based on extrapolated efficacy data from 229 adult patients in the randomized, multicenter, open-label, active-controlled, phase III NETTER-1 trial that secured the approval for the same indication in adults.
Safety analyses showed that pediatric patients experienced side effects similar to adult patients. The FDA has issued a post-marketing requirement to monitor the long-term safety of pediatric patients treated with lutetium Lu 177 dotatate.
The recommended dose for this treatment is 7.4 GBq (200 mCi) every eight weeks for four doses.
GEP-NETs are neuroendocrine tumors that occur in the gastrointestinal tract or the pancreas. They form from neuroendocrine cells—cells that make hormones, such as those that aid in the process of digestion. Worldwide, around 5.25 out of every 100,000 people are diagnosed with a GEP-NET each year.
The FDA rendered its decision on April 23, 2024.
