Full Approval for Endometrial Cancer Immunotherapy
The FDA approved the immune checkpoint inhibitor dostarlimab-gxly to treat advanced uterine cancer with certain mutations.
The U.S. Food and Drug Administration (FDA) has approved dostarlimab-gxly (Jemperli) for the treatment of adult patients with recurrent or advanced endometrial cancer that has a DNA repair defect called mismatch repair deficiency (dMMR) and has progressed during or following a treatment regimen containing platinum chemotherapy. The treatment is approved for patients who are not eligible for surgery or radiation.
The FDA previously granted accelerated approval to dostarlimab-gxly for this indication in April 2021. Accelerated approval means that continued approval may be contingent upon a confirmatory trial.
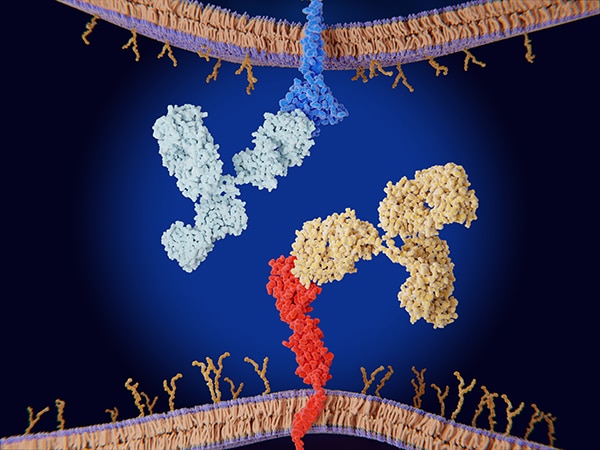
Dostarlimab-gxly is a type of immunotherapy called an immune checkpoint inhibitor, which releases the immune system’s natural brakes to help T cells fight cancer. The target of dostarlimab-gxly is the protein PD-1, an inhibitory protein on the surface of T cells that prevents them from working when PD-1 binds to its partner, PD-L1, on the surface of tumor cells. Dostarlimab-gxly blocks this interaction, keeping the T cell active against the tumor.
Mismatch repair deficiency is caused by mutations in cancer cells that prevent them from fixing errors that arise during DNA replication. In some cancer types, such as endometrial cancer, tumors with dMMR are more likely to respond to immunotherapy.
The full approval is based on a subset of patients from GARNET, a multicenter, multicohort, open-label trial. Dostarlimab-gxly was given to 141 patients with recurrent or advanced endometrial cancer with dMMR that progressed during or following treatment with platinum-based chemotherapy. Patients who had previously received an immune checkpoint inhibitor were excluded from the study.
The overall response rate was 45.4 percent, including complete responses in 15.6 percent of patients and partial responses in 29.8 percent of patients. Over 85 percent of patients had a response lasting 12 or more months, and 54.7 percent of patients had a response lasting 24 or more months.
Endometrial cancer is the most common type of uterine cancer. It forms from the cells that line the uterus, and approximately 25 to 30 percent of endometrial cancers have dMMR. According to federal statistics, it was estimated that over 65,000 individuals would be diagnosed with uterine cancer, and 12,550 patients would die of the disease in the U.S. in 2022.
The FDA rendered its decision on February 9, 2023.