Expanding the Use of an Immunotherapeutic to Liver Cancer
The FDA decision means the immune checkpoint inhibitor nivolumab is now approved for eight types of cancer.
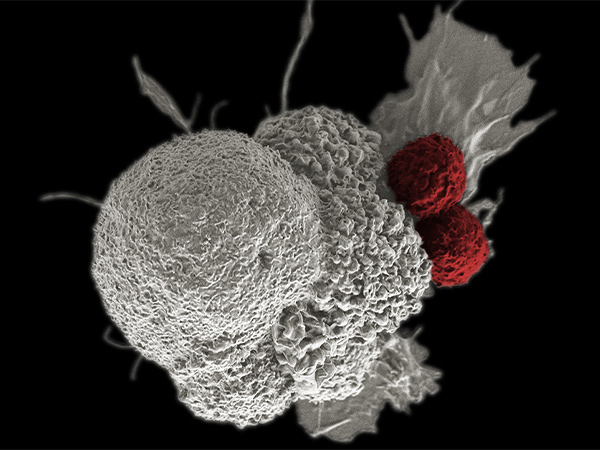
Nivolumab (Opdivo) is a type of immunotherapeutic called a checkpoint inhibitor. Its approved uses were recently expanded by the U.S. Food and Drug Administration (FDA) to include the treatment of certain patients with liver cancer.
Under the expanded approval, nivolumab can now be used for treating patients with hepatocellular carcinoma – the most common form of primary liver cancer diagnosed in the United States – that has progressed despite treatment with sorafenib (Nexavar). Sorafenib was approved by the FDA for treating advanced hepatocellular carcinoma in 2007.
With this decision, nivolumab is now an approved treatment for certain patients with bladder cancer, colorectal cancer, head and neck cancer, Hodgkin lymphoma, kidney cancer, liver cancer, lung cancer, and melanoma.
Liver cancer incidence and death rates have been increasing in the United States for the past four decades, according to data from the National Cancer Institute. In 2017 alone, it is estimated that 40,710 U.S. adults will be diagnosed with liver and intrahepatic bile duct cancer and that 28,920 will die from the disease.
The rise in death rates is so dramatic that a study published in 2014 in the AACR’s journal Cancer Research projected that liver cancer will surpass breast cancer, prostate cancer, and colorectal cancer to become the third leading cause of cancer death in the United States in 2030 if we do not develop new approaches to preventing, detecting, diagnosing, and treating the disease.
The FDA announcement stated that the approval of nivolumab was based on results from a subgroup of 154 patients enrolled in the phase I/II CheckMate-040 clinical trial. Among these patients, three saw their tumors shrink completely and 19 had partial tumor shrinkage, giving an overall response rate of 14.3 percent. Of note, 91 percent of these patients had responses that lasted six months or longer and 55 percent had responses that lasted 12 months or longer.
Importantly, the approval of nivolumab centers on response data, rather than overall survival. Thus, the company that manufactures nivolumab (Bristol-Myers Squibb) is required by the FDA to conduct additional studies to confirm that the immunotherapeutic does in fact improve survival for patients with advanced hepatocellular carcinoma.
The FDA approval was rendered on September 22, 2017.