New Therapy for Rare Sarcoma
The U.S. FDA approved the first treatment for malignant perivascular epithelioid cell tumor
The U.S. Food and Drug Administration (FDA) approved sirolimus protein-bound particles for injectable suspension (albumin-bound) (Fyarro) to treat adult patients with locally advanced, unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa).
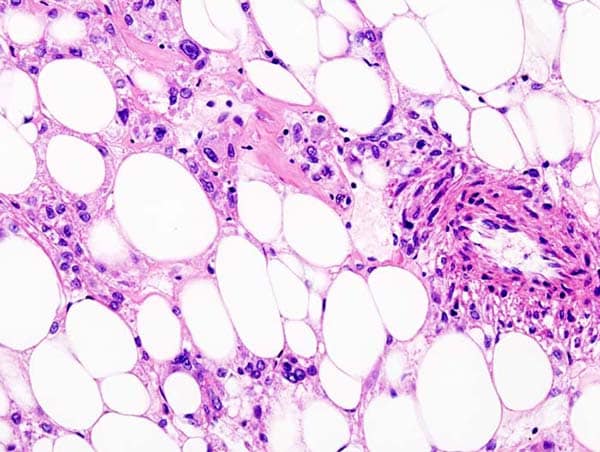
Malignant PEComas frequently harbor genetic mutations that result in the activation of the mTOR pathway, which leads to tumor growth. Sirolimus is a medication that blocks the mTOR pathway to suppress tumor growth and modulate the immune response. In this formulation, sirolimus is bound to the protein albumin, allowing sirolimus to accumulate to higher levels in solid tumors.
The approval was based on the results of a multicenter, single-arm clinical trial conducted in 31 patients with locally advanced unresectable or metastatic malignant PEComa for which surgery was not a recommended option. Patients received sirolimus protein-bound particles on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxicity.
Thirty-nine percent of patients had a partial or complete response to the treatment, including two patients with complete responses. Among the patients whose disease responded to treatment, 67 percent had a response lasting 12 months or longer and 58 percent had a response lasting longer than 24 months.
PEComa is a very rare form of soft tissue sarcoma that can form in different organs, including the stomach, intestines, lungs, female reproductive organs, and genitourinary organs. Most PEComas are benign but malignant forms are aggressive and have a poor prognosis and few treatment options.
The FDA decision was rendered on November 22, 2021.