
November 10: The Week in Cancer News
Sun exposure is an occupational hazard around the globe, and a dormant virus can cause immunotherapy complications.


Sun exposure is an occupational hazard around the globe, and a dormant virus can cause immunotherapy complications.
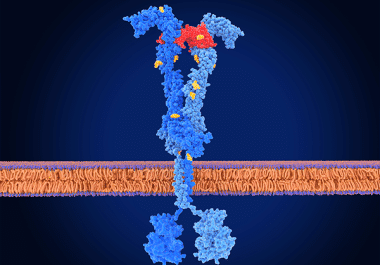
The FDA has approved another inhibitor of the VEGF receptor family for patients with metastatic, heavily pretreated colorectal cancer. The U.S. Food and Drug Administration (FDA) has approved fruquintinib (Fruzaqla) for adult patients with...
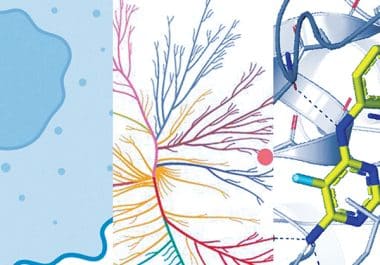
Cancers are driven by unique genetic alterations, so what if we targeted these alterations to treat the cancer? It seems straightforward, but it’s easier said than done.
New recommendation suggests ongoing lung cancer screening for those with heavy smoking history even after quitting, and more.
In the opening keynote session of the AACR Special Conference on Pancreatic Cancer, which took place September 27-30 in Boston, co-chair Jen Jen Yeh, MD, exemplified the excitement and passion exuding from the researchers...
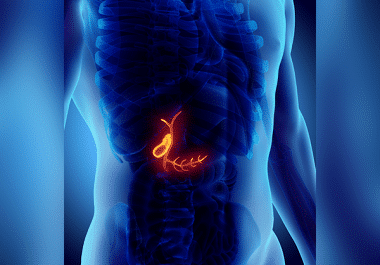
The FDA has approved the immune checkpoint inhibitor pembrolizumab for certain advanced cancers of the biliary tract. The U.S. Food and Drug Administration (FDA) has approved pembrolizumab (Keytruda), in combination with gemcitabine (Gemzar) and...

For our readers who enjoy learning about the newest tools and strategies in cancer research, the second installment of our series, “From the Bench,” is here. (Check out the inaugural post here.) This quarter,...
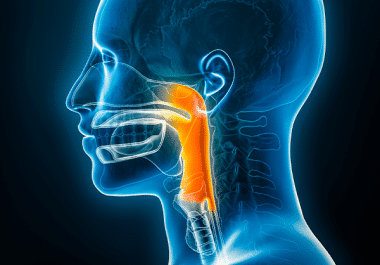
The FDA has approved the immune checkpoint inhibitor toripalimab for some patients with nasopharyngeal carcinoma. The U.S. Food and Drug Administration (FDA) has approved toripalimab-tpzi (Loqtorzi), in combination with the chemotherapies gemcitabine (Gemzar) and...

As you prepare for trick-or-treating, check out the articles selected by the editors of the 10 AACR journals for the month of October. Highlights include studies on the regulation of electrical activity between melanoma...
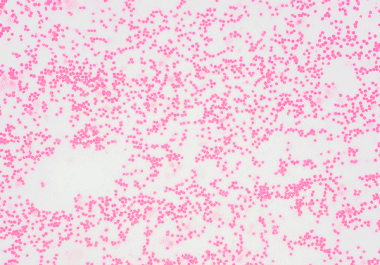
The FDA has approved the IDH1 inhibitor ivosidenib for a group of blood cancers harboring an IDH1 mutation. The U.S. Food and Drug Administration (FDA) has approved ivosidenib (Tibsovo) for the treatment of relapsed...