March 19: The Week in Cancer News
A study indicates breast cancer centers often recommend earlier and more frequent screening than national guidelines, and more news of the week from Cancer Today.


A study indicates breast cancer centers often recommend earlier and more frequent screening than national guidelines, and more news of the week from Cancer Today.
With the August 2020 U.S. Food and Drug Administration approvals of the Guardant 360 CDx and FoundationOne Liquid CDx liquid biopsy tests to detect genetic abnormalities over multiple tumor types, the use of liquid...
Lung cancer screening recommendations expand to include more smokers, and more news of the week from Cancer Today.
A patient advocate and researcher argue that the U.S. has set insufficiently ambitious cervical cancer screening goals, and more news of the week from Cancer Today.

Nonprofits provide support to young cancer patients in need of fertility preservation, and more news of the week from Cancer Today.

Organizations call to prioritize cancer patients for COVID-19 vaccination, researchers analyze rates of “low-value” breast surgeries, and UCLA cancer survivors write letters to patients undergoing treatment.
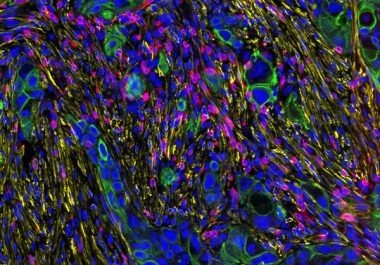
The first AACR virtual meeting of 2021, held Jan. 11-12, focused on the tumor microenvironment, the complex framework of immune cells, blood vessels, fibroblasts, and other cell types that surround a tumor and can...
An FDA committee votes to recommend holding off on approval of an immunotherapy for early-stage triple-negative breast cancer, and more news of the week from Cancer Today.
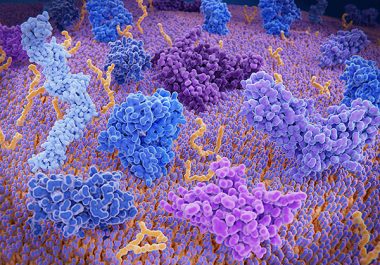
Trial results raise questions about two immune checkpoint inhibitors approved for treatment of some people with advanced breast cancer.

Fecal transplants may improve immunotherapy responses, and more news of the week from Cancer Today.