A New Treatment for Advanced Lung Cancer
The FDA has approved an immune checkpoint inhibitor – a type of cancer immunotherapy – as a first-line therapeutic for certain patients with non-small cell lung cancer.


The FDA has approved an immune checkpoint inhibitor – a type of cancer immunotherapy – as a first-line therapeutic for certain patients with non-small cell lung cancer.
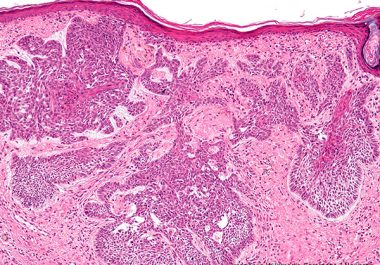
The FDA approved the immune checkpoint inhibitor, cemiplimab-rwlc, for certain patients with locally advanced or metastatic basal cell carcinoma
The FDA approved a CAR T-cell therapy – a class of cancer immunotherapy – to treat certain patients with large B-cell lymphoma, the most common form of non-Hodgkin lymphoma.
The FDA granted accelerated approval to a molecularly targeted therapeutic for certain patients with marginal zone lymphoma or follicular lymphoma.
The FDA granted accelerated approval to a new oral targeted therapeutic to treat certain adult patients with the most common form of lung cancer.
The FDA approved the combination of an immune checkpoint inhibitor and a therapeutic that prevents tumors from growing blood vessels to treat certain patients with renal cell carcinoma. The U.S. Food and Drug Administration...
The FDA approved a molecularly targeted therapeutic for treating certain patients with some rare cancers affecting the gastrointestinal system. The U.S. Food and Drug Administration (FDA) has approved the molecularly targeted therapeutic fam-trastuzumab deruxtecan-nxki (Enhertu) to treat adult...
The FDA approved a molecularly targeted therapeutic to treat certain pediatric patients and young adults with anaplastic large-cell lymphoma, a rare form of non-Hodgkin lymphoma.
The FDA approved the first once-a-day pill to lower the levels of testosterone in certain patients with prostate cancer. The U.S. Food and Drug Administration (FDA) has approved the first oral gonadotropin-releasing hormone (GnRH)...
The FDA approved the molecularly targeted therapeutic selinexor to be used in combination with another targeted agent and a synthetic corticosteroid to treat certain patients with multiple myeloma. The U.S. Food and Drug Administration...