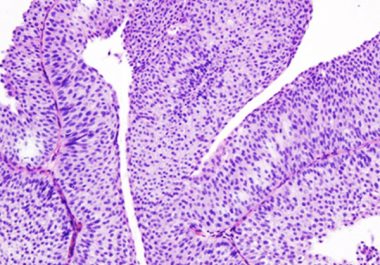
Targeting the Most Common Form of Bladder Cancer
The FDA approved a molecularly targeted therapeutic for patients with advanced or metastatic urothelial cancer.


The FDA approved a molecularly targeted therapeutic for patients with advanced or metastatic urothelial cancer.
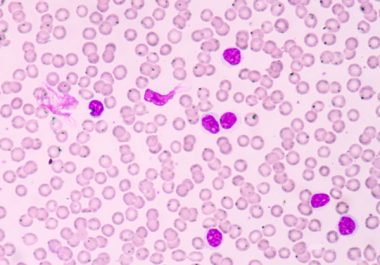
The FDA approved a molecularly targeted therapy to treat patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
The FDA approved zanubrutinib to treat patients with mantle cell lymphoma, an aggressive type of non-Hodgkin lymphoma. The U.S. Food and Drug Administration (FDA) recently approved the molecularly targeted therapeutic zanubrutinib (Brukinsa) for the...
The FDA approved the immune checkpoint inhibitor pembrolizumab in combination with the molecularly targeted therapeutic lenvatinib for women with advanced endometrial cancer.
The FDA has approved a molecularly targeted therapeutic for treating certain adults with a rare blood cancer called myelofibrosis.

The molecularly targeted therapeutic entrectinib is the second therapeutic of its kind approved by the FDA for use based on tumor biomarker rather than the site where the cancer originated. The U.S. Food and...
The FDA approved the molecularly targeted therapeutic entrectinib to treat certain patients with metastatic non–small cell lung cancer. Learn more.
A molecularly targeted therapeutic has been approved by the FDA for the treatment of tenosynovial giant cell tumors. Learn more.
The FDA approved pembrolizumab, in immunotherapy called a checkpoint inhibitor, to treat certain patients with esophageal cancer.
A new antihormone agent has been approved to treat men with nonmetastatic prostate cancer that has stopped responding to standard antihormone treatments.