New Immunotherapeutic Approved for Common Skin Cancer
The FDA approved a new immunotherapeutic for treating patients with a type of skin cancer called cutaneous squamous cell carcinoma.


The FDA approved a new immunotherapeutic for treating patients with a type of skin cancer called cutaneous squamous cell carcinoma.

The FDA expanded the use of nivolumab to include treating certain patients with small cell lung cancer.
The FDA approved a new molecularly targeted therapeutic for treating certain patients with a rare form of leukemia called hairy cell leukemia.
The FDA expanded the use of a molecularly targeted therapeutic to treat patients with the most common type of liver cancer, hepatocellular carcinoma.
The FDA approved a combination of a molecularly targeted therapeutic and an immunotherapy for treating patients with Waldenström's macroglobulinemia.

The FDA approved a targeted radiotherapeutic for treating certain patients with two types of neuroendocrine tumors.
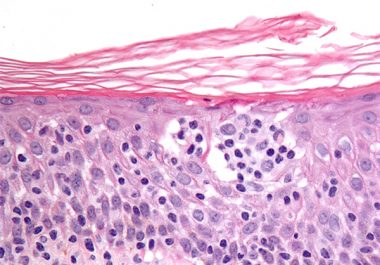
The FDA approved a new immunotherapeutic for treating certain patients with two rare types of non-Hodgkin lymphoma.
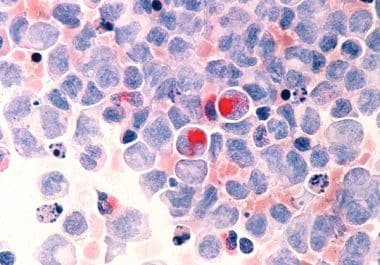
The FDA approved a new molecularly targeted therapeutic for treating certain patients with acute myeloid leukemia (AML).
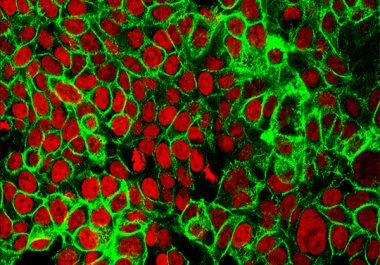
The FDA approved a combination of two immunotherapeutics for treating patients with colorectal cancer.
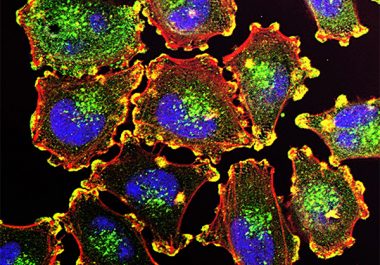
The FDA approved two molecularly targeted therapeutics for use in combination to treat patients with metastatic melanoma.