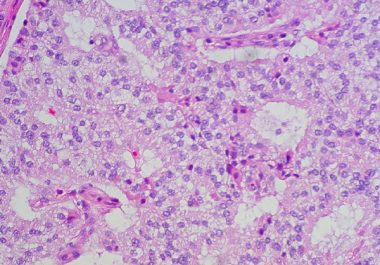
Targeted Radiotherapy for Neuroendocrine Tumors
The FDA approved a new anticancer therapeutic for treating certain patients with neuroendocrine tumors.


The FDA approved a new anticancer therapeutic for treating certain patients with neuroendocrine tumors.

The FDA approved pembrolizumab for patients with recurrent or metastatic cervical cancer that tests positive for PD-L1 and that has progressed despite chemotherapy.
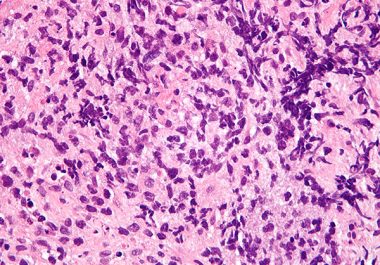
The FDA expanded the use of the immunotherapy pembrolizumab for certain patients with an aggressive type of non-Hodgkin lymphoma.
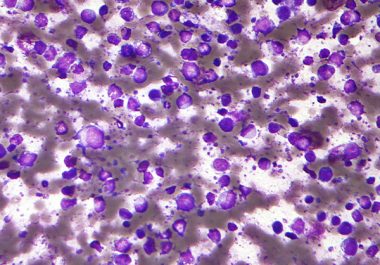
The FDA expanded the use of the CAR T-cell therapy tisagenlecleucel to include certain patients with non-Hodgkin lymphoma.
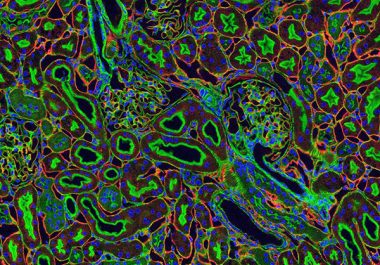
The FDA approved a combination of the immunotherapeutics to treat certain patients diagnosed with the most common form of kidney cancer.
The FDA approved a combination of molecularly targeted therapeutics for use in combination to treat patients with metastatic non-small cell lung cancer.

The May 2018 FDA approval of the immunotherapeutic durvalumab makes it the first treatment approved to reduce the risk of stage 3 lung cancer progressing.
The FDA approved a hormone therapy for treating men with nonmetastatic prostate cancer that has stopped responding to standard hormone therapy.
The FDA expanded the use of a molecularly targeted therapeutic to include the prevention of bone complications in patients with multiple myeloma.
The FDA approved a molecularly targeted therapeutic for treating certain patients who have breast cancer that tests positive for a cancer-associated BRCA1 or BRCA2 mutation.