Expanding Molecularly Targeted Therapy to Additional Types of Lymphoma
The FDA expanded the use of a targeted therapy to include certain patients with two types of non-Hodgkin lymphoma.


The FDA expanded the use of a targeted therapy to include certain patients with two types of non-Hodgkin lymphoma.
The FDA approved a molecularly targeted therapeutic for adults with mantle cell lymphoma that has progressed despite at least one prior treatment.
The FDA approved the second of a revolutionary new type of immunotherapy known as chimeric antigen receptor (CAR) T-cell therapy.
The FDA approved a molecularly targeted therapy for treating patients with a certain form of breast cancer.
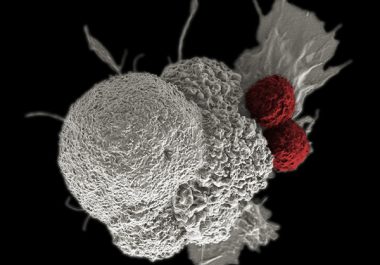
The FDA expanded an immunotherapeutic to used for treating patients with hepatocellular carcinoma – the most common form of primary liver cancer.
The FDA expanded the use of an immunotherapeutic to include the treatment of certain patients with stomach cancer (gastric cancer).
The FDA approved a molecularly targeted therapy for treating certain adults with follicular lymphoma, a form of non-Hodgkin lymphoma.
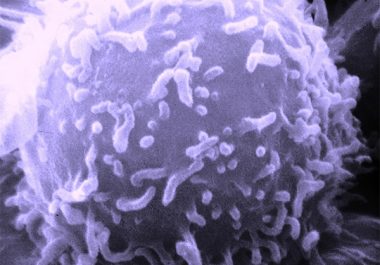
The FDA has approved a CD22-targeted antibody-drug conjugate for treating certain adults with ALL. The U.S. Food and Drug Administration (FDA) recentlyapproved a new molecularly targeted therapeutic called inotuzumab ozogamicin (Besponsa) for treating certain...
Treatment approved for the treatment of adults with two types of acute myeloid leukemia that have particularly poor prognoses.

Enasidenib is the second molecularly targeted therapeutic approved by the FDA for treating AML in recent months. The U.S. Food and Drug Administration (FDA) recentlyapproved the molecularly targeted therapeutic enasidenib (Idhifa) for treating certain...