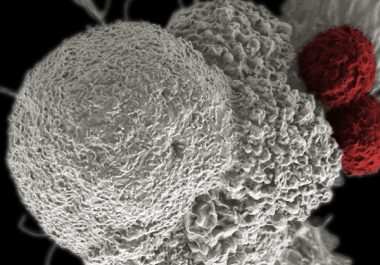
Immunotherapeutic Approved for Fourth Cancer Type
The FDA expanded the use of the immunotherapeutic pembrolizumab to include the treatment of certain patients with Hodgkin lymphoma.


The FDA expanded the use of the immunotherapeutic pembrolizumab to include the treatment of certain patients with Hodgkin lymphoma.
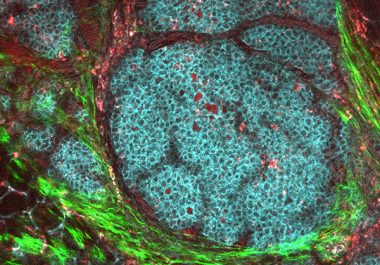
A molecularly targeted therapeutic was approved for use in combination with any aromatase inhibitor for the treatment of postmenopausal women with a certain form of breast cancer.
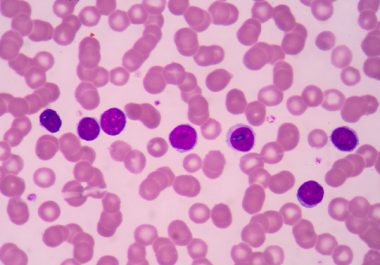
The FDA expanded the use of a targeted therapy for a form of non-Hodgkin lymphoma called marginal zone lymphoma.
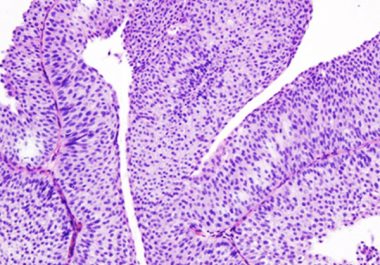
An immune checkpoint inhibitor – a form of immunotherapy – has had its use expanded for use in the treatment of bladder cancer.
The FDA approved a molecularly targeted therapeutic for women with advanced ovarian cancer linked to a BRCA gene mutation.
The U.S. FDA decision to approve nivolumab for head and neck cancer means nivolumab is now an approved treatment for five types of cancer.
The FDA approved a molecularly targeted therapeutic for treating certain patients with soft tissue sarcoma.
The FDA expanded the use of an immunotherapy to include the treatment of certain patients with lung cancer.
The FDA expanded the use of an immunotherapy to include the treatment of certain patients with head and neck cancer.

The FDA approved a liquid biopsy – a test that uses a blood sample – to identify patients with metastatic non-small cell lung cancer.