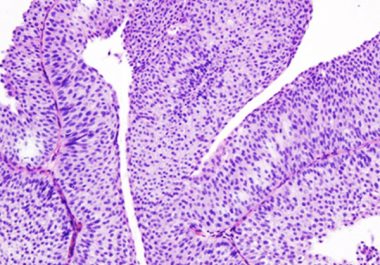
New Immunotherapeutic Approved for Bladder Cancer
The May 2016 approval of atezolizumab provided the first new treatment in more than 30 years for patients with the most common type of bladder cancer.


The May 2016 approval of atezolizumab provided the first new treatment in more than 30 years for patients with the most common type of bladder cancer.
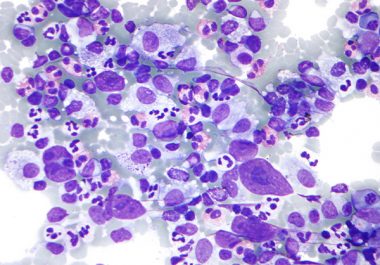
The FDA expanded the use of an immunotherapeutic to include certain patients with Hodgkin lymphoma. Learn more about this approval.
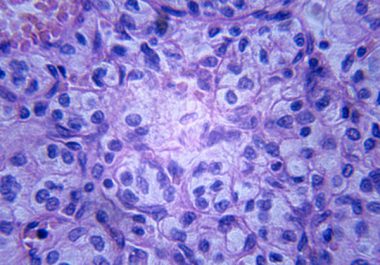
The FDA added lenvatinib to the armamentarium for oncologists treating certain patients with renal cell carcinoma.

The FDA approved a targeted therapeutic to treat patients with metastatic squamous non-small cell lung cancer.
The FDA approved cabozantinib for treating certain patients with the most common form of kidney cancer diagnosed in U.S. adults—renal cell carcinoma.
The FDA approved a new colorectal cancer screening test that uses blood samples to detect tumors.
The FDA approved a treatment for certain patients with chronic lymphocytic leukemia (CLL).

The FDA approved the use of crizotinib for treating patients with advanced non-small cell lung cancer that tests positive for a mutation in the ROS-1 gene.
The FDA expanded the use of an immunotherapy to treat certain patients with follicular lymphoma.

The FDA has approved eribulin mesylate for the treatment of one of the most common types of soft tissue sarcoma, liposarcoma.