More Lung Cancer Patients Get Additional Immunotherapy Option
The FDA has expanded the use of an immune checkpoint inhibitor for the treatment of patients with lung cancer.


The FDA has expanded the use of an immune checkpoint inhibitor for the treatment of patients with lung cancer.

The FDA has approved a targeted therapy for certain patients with lung cancer.
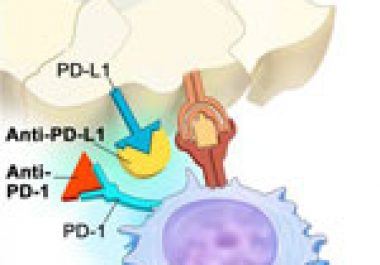
The FDA has expanded the use of pembrolizumab to include patients with advanced lung cancer that has progressed and whose tumors test positive for the protein PD-L1.
A new combination of cancer immunotherapeutics has been approved by the FDA for treating certain patients with melanoma.
A new combination of drugs was approved by the U.S. FDA for treating certain patients with advanced colorectal cancer whose disease has worsened despite treatment with chemotherapy and a biological therapy.

The FDA's approval of a targeted therapeutic called lenvatinib provides another option for patients certain forms of thyroid cancer.

The FDA has approved of a treatment for Waldenström's macroglobulinemia, a rare form of non-Hodgkin lymphoma. Learn more.
The FDA recently approved new therapeutics for acute lymphoblastic leukemia, metastatic melanoma, and advanced ovarian cancer. In December 2014, the U.S. Food and Drug Administration (FDA) issued three approvals for new medications to treat...
The FDA recently approved a new HPV vaccine that could prevent up to 90 percent of cervical cancers in the U.S. In late 2014, the U.S. Food and Drug Administration (FDA) announced the approval...