
Genomic Profiling of Children May Lead to New Treatments
Pediatric patients whose cancer had relapsed were successfully matched to new gene-targeted therapies in large European trial, according to a study in the AACR journal Cancer Discovery.


Pediatric patients whose cancer had relapsed were successfully matched to new gene-targeted therapies in large European trial, according to a study in the AACR journal Cancer Discovery.
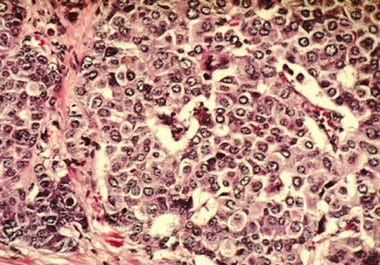
The FDA approved olaparib for patients with breast cancer who have inherited BRCA mutations and have received prior neoadjuvant or adjuvant chemotherapy The U.S. Food and Drug Administration (FDA) has approved the PARP inhibitor...
FDA approves neoadjuvant immunotherapy treatment for lung cancer, and radiation might not be needed for low-risk thyroid cancer.
The FDA approved nivolumab in combination with chemotherapy for use before surgery in certain patients with non-small cell lung cancer The U.S. Food and Drug Administration (FDA) has approved the immune checkpoint inhibitor...
Study finds lower rates of screening-related breast cancer overdiagnosis and other cancer research news of the week.
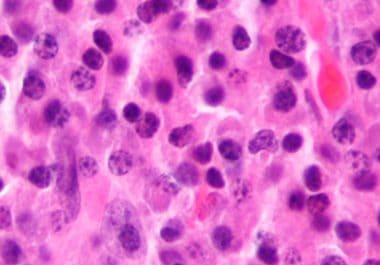
The FDA recently approved the second cellular immunotherapy to treat certain adult patients with multiple myeloma. The U.S. Food and Drug Administration (FDA) has approved ciltacabtagene autoleucel (Carvykti) to treat adult patients with relapsed...

Screening program reduces disparities in colorectal cancer, and seeing the challenges of getting cancer care through one woman’s story.

Aspirin is ineffective in preventing breast cancer recurrence, and immunotherapy holds promise for some patients with advanced anal cancer.

A new report from the American Association for Cancer Research explores how the COVID-19 pandemic affected cancer patients, researchers and care providers.

Editor’s note: This story, written by Anna Azvolinsky, first appeared in Leading Discoveries, a magazine and website that raises awareness of the AACR and its members. To learn more about Dr. Carpten’s involvement with AACR...