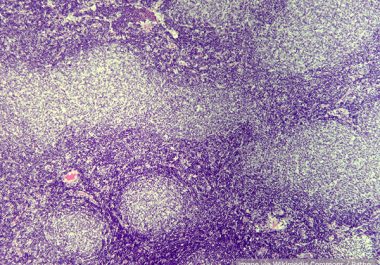
Targeting Two Types of Lymphoma
The FDA granted accelerated approval to a molecularly targeted therapeutic for certain patients with marginal zone lymphoma or follicular lymphoma.


The FDA granted accelerated approval to a molecularly targeted therapeutic for certain patients with marginal zone lymphoma or follicular lymphoma.
On December 31, 2019, the first cases of “a pneumonia of unknown cause”—now known to be COVID-19—were reported in China, and the initial confirmed case in the United States was reported just weeks later. Less than one year after this novel disease was...
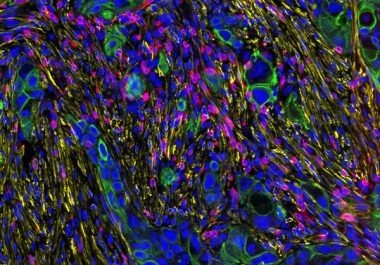
The first AACR virtual meeting of 2021, held Jan. 11-12, focused on the tumor microenvironment, the complex framework of immune cells, blood vessels, fibroblasts, and other cell types that surround a tumor and can...
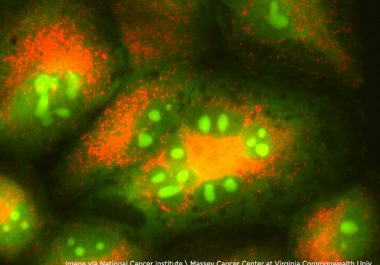
The FDA granted accelerated approval to a new oral targeted therapeutic to treat certain adult patients with the most common form of lung cancer.
An FDA committee votes to recommend holding off on approval of an immunotherapy for early-stage triple-negative breast cancer, and more news of the week from Cancer Today.
Trial results raise questions about two immune checkpoint inhibitors approved for treatment of some people with advanced breast cancer.

Fecal transplants may improve immunotherapy responses, and more news of the week from Cancer Today.

Increasing representation in the biomedical workforce would have many benefits, including reducing implicit biases and cultural incompetency in the treatment of minority patients with cancer, as well as ensuring the inclusion of diverse perspectives and enhancing innovation and creativity in cancer research.
The AACR is proud to support World Cancer Day, an annual initiative led by the International Union for Cancer Control.
The FDA approved the combination of an immune checkpoint inhibitor and a therapeutic that prevents tumors from growing blood vessels to treat certain patients with renal cell carcinoma. The U.S. Food and Drug Administration...