Expanding Molecularly Targeted Therapy to Additional Types of Lymphoma
The FDA has expanded the use of a CD30-targeted antibody?drug conjugate to include two additional types of lymphoma.
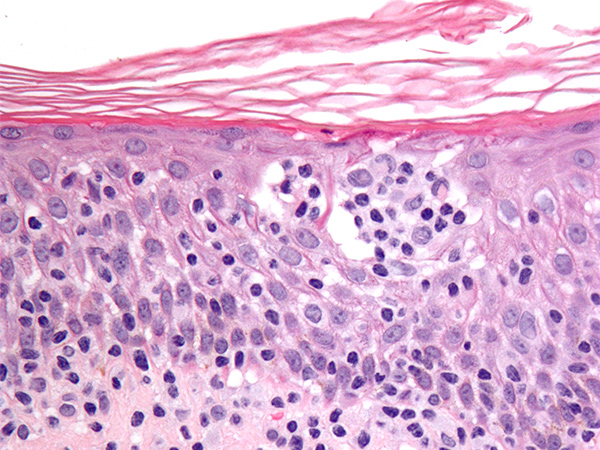
The U.S. Food and Drug Administration (FDA) recently approved expanding the use of the molecularly targeted therapeutic brentuximab vedotin (Adcetris) to include certain patients with two types of non-Hodgkin lymphoma.
Brentuximab vedotin is a type of molecularly targeted therapeutic known as an antibody-drug conjugate. The new approval is for adults who have primary cutaneous anaplastic large cell lymphoma or CD30-expressing mycosis fungoides that has progressed despite prior systemic therapy.
Brentuximab vedotin has previously been approved by the FDA for treating certain patients with classical Hodgkin lymphoma and certain patients with a type of non-Hodgkin lymphoma called systemic anaplastic large cell lymphoma.
Primary cutaneous anaplastic large cell lymphoma and CD30-expressing mycosis fungoides are types of cutaneous T-cell lymphoma. Cutaneous T-cell lymphomas are types of non-Hodgkin lymphoma that arise in immune cells called T cells. In these diseases, the cancerous T cells affect the skin, resulting in an itchy, red rash that can thicken or form a tumor. Cutaneous T-cell lymphomas are rare types of cancer; they account for less than 5 percent of non-Hodgkin lymphoma cases diagnosed each year in the United States, according to the American Cancer Society.
As for the prior types of lymphoma for which brentuximab vedotin has been approved, the cancerous cells in nearly all cases of primary cutaneous anaplastic large cell lymphoma and many cases of mycosis fungoides have on their surface a molecule called CD30.
Brentuximab vedotin targets cells with CD30 on their surface.
Antibody-drug conjugates use an antibody to deliver an attached cytotoxic agent directly to those cancer cells that display the antibody’s target on their surfaces. The precision of antibody targeting reduces the side effects of the cytotoxic agent compared with traditional systemic delivery.
In the case of brentuximab vedotin, the cytotoxic agent monomethyl auristatin E is attached to a CD30-targeted antibody using a linker. When the antibody attaches to CD30 on the surface of cutaneous T-cell lymphoma cells, the antibody-drug conjugate is internalized by the cells. This leads to monomethyl auristatin E being released from the linker and antibody. Once free, it is toxic to the lymphoma cells, which ultimately die.
The recent approval of brentuximab vedotin was based on results from the randomized phase III ALCANZA clinical trial, which were published earlier this year in The Lancet. These data showed that 56.3 percent of the 66 patients who received with brentuximab vedotin had either partial or complete tumor shrinkage for at least four months compared with just 12.5 percent of the 65 patients who received the physician’s choice of methotrexate or bexarotene. In addition, median progression-free survival was significantly greater among those who received brentuximab vedotin compared with those who received physician’s choice of treatment, 17.2 months versus 3.5 months.
Numerous additional types of lymphoma are characterized by the presence of CD30 on the surface of the cancerous cells. Thus, brentuximab vedotin is being evaluated as a potential treatment for these diseases in several ongoing clinical trials. With promising results from one of these announced recently, it is likely that we will hear more about this antibody-drug conjugate soon.
The FDA approval was rendered on November 9, 2017.
