Targeting Rare Tumors
The U.S. FDA approved a molecularly targeted therapeutic for certain patients with tumors associated with a raRe genetic disorder—von Hippel-Lindau disease.
The U.S. Food and Drug Administration (FDA) approved belzutifan (Welireg), a targeted therapy for adult patients with von Hippel-Lindau disease who require treatment for associated renal cell carcinoma, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors not needing immediate surgery.
Von Hippel-Lindau disease is a rare genetic disorder characterized by the development of cysts and tumors in various parts of the body including the kidneys, pancreas, and central nervous system. The tumors can be benign or malignant.
Belzutifan inhibits hypoxia-inducible factor-2 alpha (HIF-2α), a molecular switch that turns on expression of several genes associated with tumor growth and blood vessel formation.
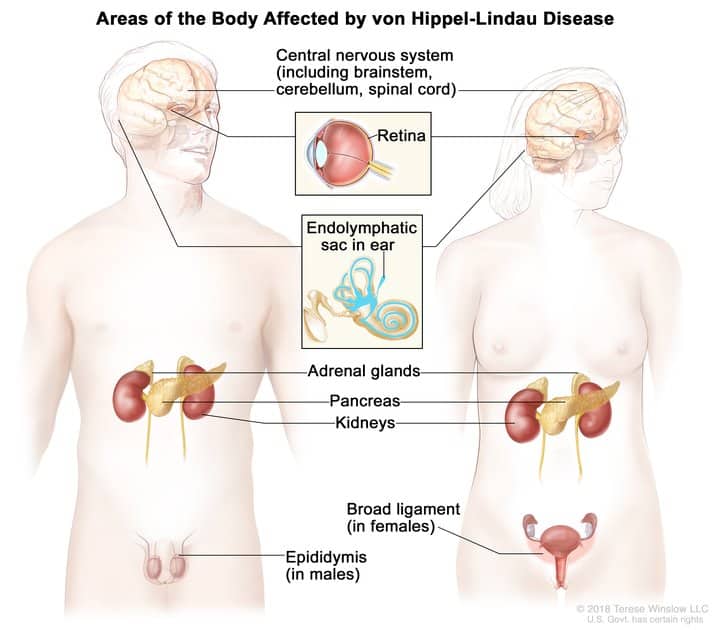
The approval of the molecularly targeted therapeutic for these patients was based on an open-label clinical trial in 61 patients with renal cell carcinoma associated with von Hippel-Lindau disease. Many enrolled patients had additional tumors associated with von Hippel-Lindau disease, allowing researchers to study the efficacy of belzutifan in central nervous system hemangioblastomas and pancreatic neuroendocrine tumors. The overall response rate was 49 percent for renal cell carcinomas, 63 percent for hemangioblastomas, and 83 percent for pancreatic neuroendocrine tumors.
Renal cell carcinoma is the most common form of kidney cancer. Pancreatic neuroendocrine tumors are a form of pancreatic cancer that account for about 5 percent of the cases diagnosed in the United States each year. Central nervous system tumors develop in the brain and/or spinal cord.
The FDA decision was rendered on August 13, 2021.
